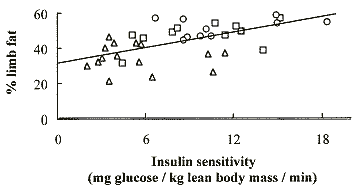Association of Severe Insulin Resistance
With Both Loss of Limb Fat and Elevated Serum Tumor Necrosis Factor Receptor
Levels in HIV Lipodystrophy
full PDF
version also available.
Dennis C. Mynarcik*;~ Margaret A. McNurlan;* Roy T. Steigbigel;* Jack
Fuhrer;* Marie C. Gelato
*Department of Medicine and ~Department of Surgery, State University
of New York at Stony Brook, Stony Brook, New York, U.S.A.
from the JOURNAL
OF ACQUIRED IMMUNE DEFICIENCY SYNDROMES 2000;25:312-321
HIV-lipodystrophy (HIV-LD) is characterized by the loss of body fat from the
limbs and face, an increase in truncal fat, insulin resistance, and
hyperlipidemia, factors placing affected patients at increased risk for vascular
disease. This study evaluated insulin sensitivity and inflammatory status
associated with HIV-LD and provides suggestions about its etiology. Insulin
sensitivity and immune activation markers were assessed in 12 control subjects
and 2 HIV-positive groups, 14 without and 15 with LD syndrome. Peripheral
insulin sensitivity (mostly skeletal muscle) was determined with the
hyperinsulinemic-euglycemic clamp. Circulating insulin-like growth factor (IGF)
binding protein-1 (IGFBP-1) and free fatty acid (FFA) levels, and their response
to insulin infusion were indicative of insulin responsiveness of liver and
adipose tissue, respectively. Serum levels of soluble type 2 tumor necrosis
factor- (TNF-) receptor (sTNFR2) were used as an indicator of immune activation.
HIV-LD study subjects had significantly reduced (twofold) peripheral insulin
sensitivity, but normal levels of FFA and reduced levels of IGFBP-1, relative to
the nonlipodystrophy groups, indicating that the loss of insulin sensitivity was
more pronounced in skeletal muscle than in liver or fat. The significant loss of
peripheral fat in the HIV-LD group (34%; p < .05) closely correlated with the
reduced peripheral insulin sensitivity (p = .0001). Levels of sTNFR2 were
elevated in all HIV-infected study subjects, but they were significantly higher
in those with lipodystrophy than without, and sTNFR2 levels strongly correlated
with the reduction in insulin sensitivity (p = .0001). Loss of peripheral fat,
normal levels of FFA, and reduced levels of IGFBP-1 indicate that insulin
resistance in HIV-LD is distinct from type 2 diabetes and obesity. The
relationship between the degree of insulin resistance and sTNFR2 levels suggests
an inflammatory stimulus is contributing to the development of HIV-associated
lipodystrophy.
Multidrug regimens have changed the character of the pathology of HIV disease
from lethal wasting, in many patients, to a managed clinical condition. However,
increased survival has also been associated with a loss of fat from the face and
the extremities and increased truncal fat, and dorsocervical fat ``buffalo
hump'' (1-3), which has been described as a lipodystrophy syndrome. This
redistribution of body fat is accompanied by metabolic perturbations including
insulin resistance and hyperlipidemia, both hypertriglyceridemia and
hypercholesterolemia (1,4), similar to metabolic syndrome X (5). The prevalence
of HIV-lipodystrophy (HIV-LD) is reported to be as high as 50% (1,6). The impact
of changes in body habitus for individuals, and the widespread occurrence
qualify this syndrome as a major cause for concern. Although many studies have
associated this syndrome with the use of HIV protease inhibitors (1,7-9), other
evidence suggests that fat redistribution is occurring in patients who have not
taken protease inhibitors (2) and, indeed, that it was occurring before the
introduction of protease inhibitors (10,11). Defining the etiology of the
HIV-associated LD syndrome and its related metabolic abnormalities is an urgent
priority.
In obesity and type 2 diabetes mellitus, two
factors have been implicated in the etiology of insulin resistance. These
factors are elevated free fatty acids (FFAs) and the cytokine tumor necrosis
factor- (TNF-). In both obesity and type 2 diabetes, insulin resistance is
associated with elevated levels of FFAs (5,12). Elevated FFA levels alone are
sufficient to induce insulin
resistance without any underlying pathology (13,14), apparently by altering
insulin signaling in skeletal muscle (15). Additional markers of insulin
resistance include increased abdominal fat in obesity (16) and type 2 diabetes
mellitus (17-19) and elevated serum levels of insulin-like growth factor binding
protein-1 (IGFBP-1) (20).
Insulin resistance in obesity has been associated with a cytokine, TNF-, which
is specifically implicated in the induction of insulin resistance (21) through
inhibition of the insulin signaling cascade that regulates glucose uptake (22).
The role of TNF- in the development of insulin resistance currently seen in HIV
disease is not known. Cytokines, such as TNF-, were suspected in the wasting
aspects of HIV infection, but low circulating levels failed to support this (23)
(see also commentary by Grunfeld [24]), whereas cytokines did contribute to
hepatic lipogenesis (25). With effective antiretroviral treatment, HIV-infected
patients have improved disease control, as assessed by mortality (26), low to
undetectable viral load, and increased numbers of CD4+ lymphocytes (27). The
components of the TNF system are reduced in patients receiving highly active
antiretroviral therapy (HAART) (28) but it would be instructive to know whether
they remain depressed in HIV LD.
The present study was designed to characterize insulin resistance manifest in
HIV patients who have the LD syndrome, but not overt diabetes, i.e., fasting,
hyperglycemia. The degree of peripheral insulin resistance in these patients was
assessed by the hyperinsulinemic-euglycemic clamp (29). This method suppresses
hepatic glucose output and provides an accurate measurement of the rate of
insulin-stimulated glucose disposal in skeletal muscle. Insulin resistance was
related to physiologic parameters known to be altered in insulin resistant
states, that is, body fat distribution (30,31), circulating levels of FFAs
(32-34), and IGFBP-1 (20). In addition, serum levels of soluble type 2 TNF-
receptor (sTNFR2) were assessed, inasmuch as elevated levels of sTNFR2 have not
only been associated with the clinical course of HIV infection (35), but in
particular were found to correlate with insulin resistance in obesity (36).
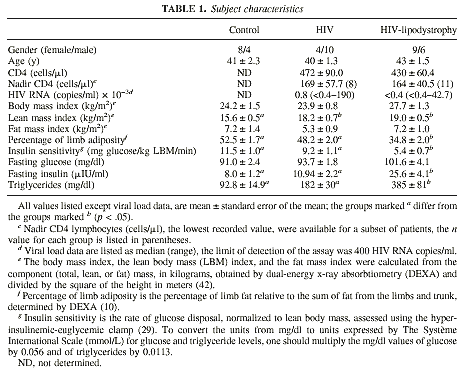
STUDY SUBJECTS AND METHODS
Those enrolled in the present study consisted of 12 healthy control study
subjects, 14 study subjects infected with HIV without LD (denoted here as HIV),
and 15 HIV-positive study subjects with the LD syndrome (HIV-LD). There were two
exclusion criteria, diabetes, based on a random plasma glucose level >200
mg/dl or a fasting plasma glucose >126 mg/dl (based on diagnostic criteria in
the Report of the Expert Committee on the Diagnosis and Classification of
Diabetes Mellitus, 1997), and acute illness within the 3 months preceding the
study. HIV-LD study subjects had self-reported loss of fat from the limbs and
face with accumulation of fat in the abdomen and trunk, which was confirmed by
physician assessment at the time of study. Control study subjects were matched
for age and gender with the HIV and the HIV-LD groups. The HIV group consisted
of 7 patients who were asymptomatic, 3 patients with AIDS, and 4 patients with
AIDS and a prior history of weight loss. The HIV-LD group consisted of 7
asymptomatic patients and 8 patients with AIDS.
Most HIV-infected study subjects were receiving multidrug regimens and continued
these medications during the study. In the HIV group, all patients except 2 were
taking nucleoside reverse transcriptase inhibitors, 2 were taking nonnucleoside
reverse transcriptase inhibitors, and 11 were taking protease inhibitors.
Similarly in the HIV-LD group, all except 1 patient were taking reverse
transcriptase inhibitors, 3 were taking nonnucleoside reverse transcriptase
inhibitors, and 13 were taking protease inhibitors. No difference was found in
the degree of peripheral fat loss or insulin resistance in those patients taking
protease
inhibitors and those who were not, although the number of patients who were not
taking protease inhibitors was small.
DISCUSSION:
Unique Because Trunk Adiposity & Insulin Resistance Appears not to be
Related to Fat Loss as in Type 2 Diabetes
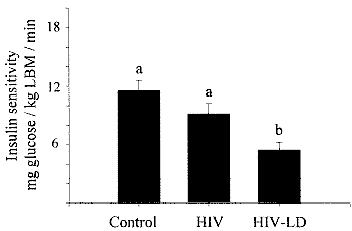
In the present study, patients with clinically defined HIV LD had a 34% reduction in
percentage of limb fat, relative to findings in the control group (Table 1).
 These These patients exhibited severe insulin
resistance (Fig. 1) of a magnitude similar to that seen with type 2 diabetes
mellitus (43,44).
These These patients exhibited severe insulin
resistance (Fig. 1) of a magnitude similar to that seen with type 2 diabetes
mellitus (43,44).
The loss of limb fat was highly
correlated (p = .0001) with insulin resistance, as shown in Figure 2,
demonstrating, for the first time, that insulin resistance accompanies the
pathologic loss of peripheral fat.
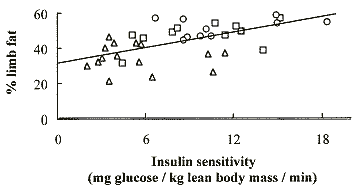
The well recognized association between trunk
adiposity and insulin resistance appears not to be a significant factor in
HIV-LD. When patients with HIV-LD were stratified into groups with the greatest
versus the least amount of trunk adipose tissue (19.0 kg versus 6.8 kg trunk
fat), the two groups had similar insulin sensitivities (data not shown). An
alarming feature of HIV-LD is that in the context of a routine clinic
examination, the HIV-LD group may be unremarkable, with normal screening glucose
levels (data not shown), and elevated triglyceride levels, a feature that has
become an expected finding of HIV infection (45). The HIV-LD patients also had
significantly elevated levels of the sTNFR2 (Fig. 3).
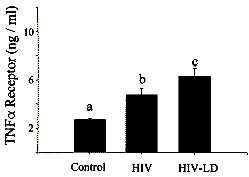
This finding is of interest, given that these
patients are doing well clinically, with well-controlled HIV replication and
improved numbers of CD4+ lymphocytes. Furthermore, insulin resistance in the
HIV-infected population was highly correlated (p = .0001) with the serum levels
of the sTNFR2 (Fig. 4), suggesting that inflammation may contribute to the
pathophysiology of LD and insulin resistance.
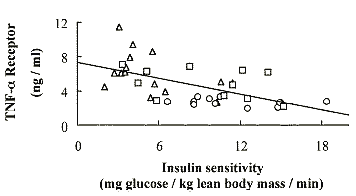
At present, the cellular source of the
sTNFR2 is unknown, and investigations are currently under way to identify this
source.
The clinical characteristics of HIV-LD bear a resemblance to the rare forms of
acquired and congenital lipodystrophies (49-52), as well as an animal model of
lipoatrophic diabetes (53). The congenital and acquired generalized
lipodystrophies are characterized by loss of both trunk and limb fat, increased
LBM (51), severe insulin resistance (52), normal levels of FFAs (51,52), and
suppressed levels of IGFBP-1 (50). All these characteristics, except loss of
trunk fat, are also shared with HIV-LD, suggesting that loss of peripheral fat
alone may be sufficient to induce a state of peripheral insulin resistance. The
increases in the LBM index of the HIV-LD group may also be causally linked to
the LD syndrome.
Fat distribution, physiologic parameters, and serum markers associated with
different insulin-resistant states are summarized in Table 2. These data
demonstrate that the insulin resistance in HIV-LD is distinct from that
associated with most forms of type 2 diabetes, based on hepatic insulin
sensitivity, reflected by both fasting glycemia (Table 1) and IGFBP-1 levels
(Fig. 5), and insulin sensitivity of adipose tissue reflected by fasting FFA
levels (Fig. 5).
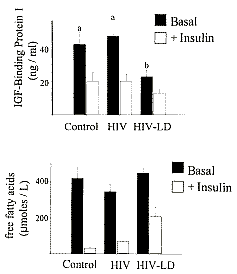
The distinction between HIV-LD and
obesity, although not so dramatic as with type 2 diabetes, is still clearly
demonstrated by the differences in fasting FFA levels (Table 2). Table 2 also
demonstrates that the insulin resistant state associated with HIV-LD has most in
common with the exceedingly rare congenital and acquired lipodystrophies (52).
The similarities include the loss of peripheral fat, increased LBM, severe
insulin resistance, normal fasting FFA levels, and reduced IGFBP-1 levels.
Although both increased trunk fat and elevated FFAs are commonly associated with
insulin resistance, the presence of insulin resistance in acquired and
congenital LD with loss of trunk fat and normal FFAs, suggests that loss of
peripheral fat alone may also be sufficient to cause insulin resistance.
A second paper by the same
author.
Insulin-like Growth Factor System in Patients With HIV Infection: Effect of
Exogenous Growth Hormone Administration
Dennis C. Mynarcik*;* Robert A. Frost;~ Charles H. Lang;* Kim
DeCristofaro;~ Margaret A. McNurlan;~ Peter J. Garlick;* Roy T. Steigbigel;*
Jack Fuhrer;± Sang Ahnn;* Marie C. Gelato
*Department of Medicine, ~Department of Surgery, and ±Department of
Preventive Medicine, State University of New York at StonyBrook, StonyBrook, New
York, U.S.A.
R. A. Frost and C. H. Lang are currently affiliated with the
Department of Cellular and Molecular Physiology, Pennsylvania State University,
Hershey Medical Center, Hershey, Pennsylvania, U.S.A.
from the JOURNAL OF ACQUIRED IMMUNE DEFICIENCY SYNDROMES
1999;22:49
Summary:
The purpose of this study was to characterize changes in the levels of
insulin-like growth factor-I (IGF-I) and IGF binding proteins (BP) 1, 2, and 3
in HIV-infected adults throughout the course of their disease, and to assess the
responsiveness of the IGF system components to growth hormone (GH)
administration (6 mg/day) for 2 weeks. Healthy control study subjects (n =10)
were compared with patients who were either HIV-positive (n = 9), had AIDS
without weight loss (n = 13), or had AIDS with >10% weight loss (n = 6), all
of whom had been free of acute illness for at least 3 months.
Under basal conditions, fasting serum
concentrations of epinephrine, norepinephrine, cortisol, glucagon, insulin, IGF-I,
and IGFBP-3 were not significantly different among the four groups. The serum
concentrations of IGFBP-1 and IGFBP-2 were significantly higher in AIDS patients
with wasting than in the other three groups (p < .05). In addition, there was
a statistically significant positive correlation between the levels of IGFBP-1
(p = .004) and IGFBP-2 (p = .03) and the stage of disease.
Following GH administration, the serum
concentrations of insulin and IGF-I were increased in all groups (p < .05).
In addition, the increases in insulin levels correlated with stage of disease (p
= .004). The responses of the IGFBPs were more variable. GH administration
significantly increased the levels of IGFBP-3 in all groups except the patients
with AIDS wasting, whereas the levels of IGFBP-1 were significantly decreased in
controls and AIDS patients. These results demonstrate that there is a continuum
of both elevations in the IGFBPs and altered metabolic responsiveness in
patients infected with HIV that increases with the severity of the disease.
These data also demonstrate that AIDS patients, who are free from secondary
infection, respond to administration of GH by significantly increasing hepatic
IGF-I production.
 These These patients exhibited severe insulin
resistance (Fig. 1) of a magnitude similar to that seen with type 2 diabetes
mellitus (43,44).
These These patients exhibited severe insulin
resistance (Fig. 1) of a magnitude similar to that seen with type 2 diabetes
mellitus (43,44).