
The newest development
in treatment for HCV is pegylated interferon. The measure for evaluating
treatment success has been achieving and maintaining undetectable HCV viral
load. However, many people are unable to achieve that goal. Histological
improvement is observed in non-responders to interferon or interferon/ribavirin
therapy. The idea is to take advantage of that improvement and try to maintain
it. Recent research has suggested that maintenance therapy of interferon may
maintain that improvement, and delay histology or hepatitis disease progression.
This observation has
given impetus to begin two large studies to test the concept of low-dose
interferon maintenance therapy. In practice, doctors have been using maintenance
therapy when they run out of the limited treatment options available. After a
patient's therapy ends, a doctor may continue with maintenance therapy if the
person did not achieve undetectable viral load. So, for those individuals who do
not respond to HCV therapy, it remains possible that maintenance therapy may
keep people alive and healthy until new treatments are available. And much
attention is indeed being paid to researching new HCV treatments. In studies of
pegylated interferon, more histologic improvement was noted in the individuals
receiving pegylated interferon than in those treated with current dosage
regimens of interferon. In fact, in a study of the Roche Pegasys, Pegylated
Interferon for compensated cirrhotics, Heathcote reported that 31% of
individuals who received IFN 3 MU three times per week had histologic
improvement (8% had sustained virologic response), and 54% had histologic
improvement with Pegasys (30% had sustained virologic response). However, for
individuals with genotype 1 in that study, the sustained virologic response was
much lower. Most coinfected individuals have genotype 1.
The latest data on both
Pegylated interferons were presented at EASL in April. Pegylation is a process
whereby polyethylene glycol (also known as PEG) is attached to the interferon
and prevents the interferon from being rapidly eliminated from the body.
Normally interferon stays in the body for about 24 hours but with pegylation it
stays in the body for 7 days at longer lasting and higher levels. This permits
once weekly injections. Currently, the only FDA approved administration of
interferon is three times weekly subcutaneous injections. However, many think
that the FDA approved dosing was inadequate because on days in between dosing
the hepatitis C virus was left to replicate without any antiviral pressure from
drug therapy. So currently, daily dosing of interferon is often used. As well,
dosing with higher levels is also practiced. The expectation is that increased
blood drug levels of pegylated interferon will increase the antiviral activity
against HCV. The preliminary data below supports this. Over the course of a week
interferon blood levels are kept high.
C Trepo reported for the
Hepatitis Interventional Therapy Group on this phase III study comparing 3 doses
of Schering-Plough's Pegylated-Intron alfa-2b. Following the first study report
is a preliminary report comparing Peg Intron+ribavirin to Peg Intron alone. This
study compares Pegylated Intron monotherapy to IFNalfa-2b 3 Million Units three
times per week. It's a randomized, double-blinded, dose finding efficacy study
of 1,200 treatment-naďve individuals with chronic hepatitis C, elevated ALT and
compensated liver disease. Study participants received 1 of 3 doses of Peg IFN
alfa-2b: 0.5 ug/kg (n=315) once weekly by subcutaneous injection, 1.0 ug/kg
(n=297) once weekly, or 1.5 ug/kg (n=304) once weekly. Or they were randomized
to the control arm of IFN alfa-2b 3 MIU three times per week (n=303).
Participants received the drug for 48 weeks and there were 24 weeks of
follow-up. A liver biopsy was performed at baseline and after 72 weeks. The
primary endpoint was a sustained loss of HCV viral load 24 weeks after treatment
stopped.
The age across all 4
treatment groups was about the same at 43 years. There were about the same
percent of men in all arms (63%). About 5% in each arm were Black. In the two
low dose Peg IFN arms there were 67% genotype 1. In the Peg IFN 1.5 ug/kg arm
there were 73% genotype 1 and 72% in the IFN alfa-2b arm. So 26-29% across all 4
arms were genotypes 2/3. Each arm had about 73% with HCV viral load >2
million.
Sustained Virologic Responses for Peg IFN Alfa-2B (at week 72) (See Table 15)

Sustained Response by Genotype & HCV Viral Load (See Table 16)
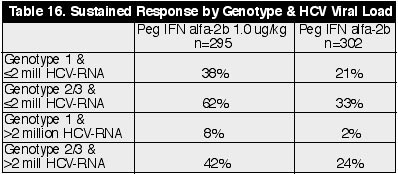
Overall, 49% and 23% at
the end of treatment and the end of follow-up, respectively, in the Peg IFN 1.5
ug/kg dose arm had HCV RNA undetectable (<100 copies/ml). In the IFN alfa-2b
MIU 3x/wk arm, 24% and 12% had undetectable HCV RNA at the end of treatment and
after follow-up. In the Peg IFN 1.0 dose arm 41% and 25% had undetectable,
respectively, at the end of treatment and after follow-up. And in the Peg 0.5
dose arm, 33% and 18% had undetectable.
White blood cell,
platelets, and neutrophil counts went down during treatment but bounced back to
normal after treatment ended. The WBC and the platelets went down a little more
in the two high Peg IFN dose arms than the low Peg dose arm and the IFN alfa-2b
arm. The difference in the platelets could be 50,000 between the arms.
Depression, irritability and other psychiatric related adverse events were the
same between arms.
Dose discontinuation was
9%, 11%, and 9% in the three Peg IFN alfa-2b dose arms (0.5, 1.0, and 1.5 ug/kg),
and 6% in the IFN alfa-2b 3 MIU 3x/wk arm, although the presenter said there was
no real difference between the arms. The discontinuations occurred more often in
the earlier parts of treatment. Discontinuations during weeks 1-24 were 4%, 7%,
6%, and 4% in the 4 arms. While discontinuations were 4%, 4%, 3%, and 2% during
weeks 24-48. Dose reduction was more in the Peg arms--9%, 14%, 15%, and 6% in
the 4 arms, respectively, but the presenter said this was due in part to more
aggressive dose reduction as part of the protocol.
Schering reported data
from a comparison of Peg-IFN 2b with IFN 2b alone. End-of-treatment response: in
the combination arm of high dose of 1.4 ug/kg Peg IFN+ribavirin 81% had
undetectable HCV RNA while 50% in the Peg IFN alone arm had undetectable. In the
0.7 Peg IFN dose combination arm 69% vs 63% had undetectable, respectively, and
in the low dose 0.35 ug/kg arm 58% in the combination arm and 50% in the Peg IFN
alone arm had undetectable.
At the end of follow-up,
60% had undetectable in the 1.4 ug/kg combination arm versus 42% in the
monotherapy arm. In the 0.7 ug/kg arm 53% vs, 44% had undetectable; and in the
0.35 dose arm 17% and 0% had undetectable.
Pegasys; PEG Interferon alfa-2a for
Chronic Hepatitis C
S Zuezem reported for
the Pegasys International Study Group on this phase III study of the safety and
efficacy of this once weekly pegylated interferon from Roche. This study
compares the efficacy and safety of Peg IFN alfa-2a administered once per week
with an induction regimen of standard IFN alfa-2a administered 3 times weekly
for 48 weeks. The primary study endpoint is undetectable HCV RNA (<100
copies/ml, Roche PCR assay), and normalized ALTs after a 24 week follow-up
period. 531 patients were randomized to either 180 ug Peg IFN alfa-2a once
weekly or to an induction regimen of 6 Million Intl. Units of IFN alfa-2a three
times weekly for 12 weeks followed by a dose of 3 MIU three times weekly for 36
weeks. A biopsy was performed at baseline and after the 72 week period.
There were 67% men in
both arms, average age 41 in both arms, 85% Caucasian in both arms, ALT 98 in
Peg arm and 94 in other arm. Total HAI score was 8.6 in Peg arm and 9.0 in other
arm. 12% had transition to cirrhosis or cirrhosis in Peg arm and 15% in other
arm. Genotype 1 63% and 61% in Peg and other arm, respectively. Genotypes 2/3
also about same in both arms. HCV viral load 7.4 log in Peg arm and 8.2 in other
arm.
At the end of follow-up
(72 weeks) 45% normalized their ALTs in Peg arm and 25% in other arm. At the end
of treatment (48 weeks), 46% and 35% normalized their ALTs in Peg and other arm,
respectively (p<0.001). The overall virological response (<100 copies/ml)
in the Peg arm was 69% at the end of treatment and 38% at the end of follow-up.
For the other arm, 28% at the end of treatment and 19% at the end of follow-up
had undetectable HCV viral load (p<0.001). At the end of follow-up 38% in the
Peg arm versus 17% in the other arm had both normalized ALT and undetectable
viral load (p<0.001). In the Peg arm, 63% had histological improvement in
their liver at the end of follow-up compared to 55% in the other arm. Histologic
improvement was defined as a decrease of at least 2 points in the Knodell
Histologic Activity Index.
Sustained Virologic Response (undetectable after 72 week follow-up) Analysis By Genotype: (See Tables 17 & 18)


Discontinuation for any
adverse event or lab abnormality was 7% in the Peg arm and 10% in the induction
arm. Dose modification for adverse event (AE) or lab abnormality was 18% in both
arms, for AE 8% and 12%, respectively, and 14% for lab abnormality in Peg arm
and 9% in induction arm--mostly due to neutropenia (decreased neutrophils)- it
was 11% in Peg arm and 7% in other arm. The side effect profile was similar for
both arms except in a few instances where the higher side effect rate occurred
in the induction arm.
Limited research and
knowledge about HCV/HIV coinfection suggests that a coinfected person may be
better off starting HCV therapy when CD4s are high--above 500 or at 800. Keep
reading. The study reviewed below was reported at Durban by Hernan Valdez from
Case Western in Cleveland and raises an interesting question. In his study,
people with HCV/HIV did not respond to HCV antigen, but people with HCV alone
did respond. Although responses to HCV antigens improved in HIV-infected persons
who were receiving antiretroviral therapy, the responses did not reach the
levels seen among HIV-negative HCV+ patients. So HAART may help improve response
to HCV, but still the response is less than for those with HCV alone. This study
suggests, as other studies have suggested, that having higher CD4 s may improve
response to HCV, as it may help immune system control HCV better. Further, I
think it may be the CD4 "repertoire" that is more important than
absolute CD4 count. HIV viral load improvements should lead to CD4 increase. The
key CD4 parameter most important for response to IFN/RBV therapy may be CD4
nadir prior to antiretroviral therapy for HIV. I would suggest that, as in HIV,
if a person has lost the CD4 "repertoire" response to a particular
antigen (in this case to HCV), maybe that will affect how the coinfected person
responds to HCV either before or after HAART. If they've lost the CD4 repertoire
for HCV, they just may not be able to respond well to HCV and they may not
respond well to IFN+RBV therapy. In Valdez's study, levels of HCV viral load
were noted to correlate with the weakness of lymphoproliferative responses to
HCV antigens. Thus, one may expect improved control of HCV with immune
restoration. So, it may be very important to identify and treat HCV in HIV
before CD4s decline.
In a related study
detailed below, a group out of Italy led by M. Pouti examined a group of 204
patients, all of whom had HCV infection. 84 of those had co-infection with HIV.
This group looked at the relationship between liver fibrosis in those with HIV
vs. those without HIV. This work has been done before by other groups, and the
consensus seems to be that HCV has a more rapid progression in the HIV
co-infected. Once again this was confirmed in this study. In addition, however,
this group analyzed the HIV positive subjects to see if low cd4Ýs in the HIV/HCV
co-infected translated in more advanced liver fibrosis. And indeed their main
finding was that more advanced liver
fibrosis, defined by the presence of stage 3 or 4 fibrosis, was associated with
immune suppression defined as <500 cells/ml and was independent of
gender, age, duration of infection (HCV), and ETOH use.
Commentary:
The cut-offs for what was termed immune suppression are rather high in this
study (<500 cd4Ýs). Still the results strongly suggest that starting HIV
antiviral therapy early in those co-infected with HIV and HCV may slow the
progression to liver fibrosis and cirrhosis. Puoti concluded antiretroviral
combination therapy that aims at keeping high CD4 counts should be regarded as a
priority in the care of HIV and HCV coinfected patients.
The information related
in this article, when taken in consideration with additional knowledge and
research, suggest that for the HCV/HIV coinfected patient, starting HCV therapy
when CD4s are high could make a significant difference in the outcome of HCV
therapy. Starting HCV therapy when a person has 500 or even 800-900 CD4s may be
beneficial because the person may not have lost their HCV-specific CD4 response
or the proliferative lymphocyte response to HCV. There is limited research
supporting this notion and certainly no or little clinical research supporting
this, but oftentimes clinical treatment precedes research. Starting HCV
treatment when CD4s are depleted, at 250 for example, may be too late to allow
for a good virologic response to HCV and a response to HCV therapy. By this time
the patient may have less capacity to mount a response to HCV or HCV therapy.
The patient may have lost his or her HCV proliferative lymphocyte or CD4
response capacity. When does the patient lose the lymphocyte proliferative
response (LPR) to HCV? In HIV, that question remains unanswered with regards to
LPR to specific pathogens or infections like PCP or CMV. In HIV there appears to
be general agreement that the LPR or HIV-specific CD4 response is lost rather
quickly after HIV infection. The same may be the case regarding HCV. If that is
the case, it may be important to consider HCV therapy as soon as possible after
learning one is HIV-positive when CD4s are high.
Immunological
responses to hepatitis C and non-hepatitis C antigens in hepatitis C virus (HCV)
infected and human immunodeficiency virus (HIV)-HCV coinfected patients
Hernan Valdez of Case
Western University in Cleveland, USA reports on this study. Vigorous HCV-specific
CD4 responses are associated with clearance of HCV viremia, but these are absent
or of low magnitude in most patients with chronic HCV infection. HIV-HCV
coinfected patients progress faster to cirrhosis and hepatocellular carcinoma
than HCV-infected subjects. Although after treating HIV with HAART HCV
progression may change. Valdez examined immune phenotype and function in HCV(+)
subjects to better characterize immune function in HCV infection in the presence
and absence of HIV infection.
Uninfected = Un (9), HCV-infected
= HCV(+) (9), HCV-HIV infected = HIV/HCV (10), HCV-HIV infected on HIV treatment
= HIV/HCV-Tx (9), and untreated HIV-infected, HCV-uninfected = HIV(+) (10)
patients had blood drawn for flow cytometry, lymphocyte proliferation and
ELISPOT assays. Entry criteria: no cirrhosis, >300 CD4 (HIV), no recent
treatment with IFN or Hepatitis B coinfection.
Patients were well
matched for age and gender. HCV infection tended to cause an increase in the
percentage of activated CD8 cells (U = 2%, HCV(+) = 6%, p = 0.1). Proliferative
responses to non-HCV antigens were comparable in HCV(+) and U subjects. A
greater proportion of HCV(+) had a stimulation index (SI) >3 to NS3 compared
to HIV/HCV and HIV/HCV-Tx (67%, 0%, 11%, p>0.006). The log SI to NS3 was
significantly higher (p>0.04, p>0.009) in HCV(+) (median, IQR 0.6,0.5)
than in HIV/HCV (0.3,0.5) or HIV/HCV-Tx (0,0.4). Among HCV-infected patients,
HCV-VL correlated directly with ALT (r = 0.52, p>0.01) and inversely with the
number of CD4+ lymphocytes (r = -0.55,p>0.008) and proliferation to NS3 (r =
-0.55,p>0.008).
Valdez concluded that
lymphocytes of HCV-infected patients fail to respond to HCV antigens while
responses to other antigens are preserved. Infection with HIV potentiates this
deficiency. Poor CD4+ T cell responses to HCV may determine the failure to
control HCV propagation.
We know that HIV enters
the brain shortly after a person is infected with HIV. It does appear as though
individuals with HIV may experience symptoms related to this, such as reduced
alertness or a slower thinking capacity due to HIV. At both recent liver
conferences (DDW and EASL), two different research groups reported research
findings suggesting that HCV in individuals with less advanced disease (non-cirrhotics
or mild fibrosis) affects the brain and reduces its functioning capacity. This
suggests that a person with both HCV and HIV may be affected even more with
regards to brain functioning. Over the years people with HIV have complained
about experiencing fatigue and/or itching. We now know that many people with HIV
also have HCV, and that HCV can cause itching and fatigue. The findings reported
at DDW and EASL suggest that HCV related fatigue may be associated with the
affect of HCV on the brain.
It's known that
individuals with advanced cirrhosis can experience hepatic encephalopothy which
can cause brain disorder, but it's important to bear in mind that the
participants in the studies discussed below did not have such advanced HCV
disease, so the brain dysfunctioning found was not due to hepatic
encephalopoathy.
At DDW, Ludwig Kramer
and a research group from the University of Austria, reported that
"cognitive processing was subclinically impaired in patients as compared to
healthy subjects." They studied the impact of HCV infection on sensitive
markers of cognitive brain function. Fifty-eight noncirrhotic patients with
chronic HCV infection (age, 45▒13 years, mean▒SD) were studied by P300
event-related potentials (an objective measure of cognitive processing) and by
the SF-36 questionnaire for assessment of health-related quality of life.
Findings were compared to 58 matched healthy subjects. He found that P300 test
results were impaired in patients with HCV compared to healthy volunteers, and
concluded that patients with chronic HCV infection in the absence of cirrhosis
exhibit a subclinical neurophysiological impairment. Cerebral function, however,
seems to normalize with antiviral treatment. Although it was not apparent to me
if normalization was tied with significant reductions in HCV viral levels, my
feeling is that improvements in cerebral function can improve with HCV treatment
despite no HCV viral level reductions. More detailed data and discussion are
available below at the end of this report.
At EASL, DM Horton
presented an oral talk on brain dysfunction in people with HCV for a UK research
group from the Imperial College School of Medicine and St Mary's Hospital in
London. First he reviewed two studies. He mentioned a UK study (Foster et al
1998) using the SF-36 questionnaire, and reported people with HCV compared to
normal controls scored worse in physical and social functioning, energy and
fatigue, and other measures. These results were independent of intravenous drug
use. In a large US (Johnson et al 1998), 309 IVDUs both with or without HCV were
tested for depression and those with HCV (57.2%) were found to have
significantly more depressive symptomology than those who were negative to
hepatitis (48.2%).
In an attempt to further
define this neuropsychological syndrome, they administered a battery of
neuropsychometric tests to 15 patients with histologically mild hepatitis C from
liver biopsy. They tested for attention (included: simple reaction time, choice
reaction time), working memory (numeric & spatial working memory), and
secondary memory (delayed word recall). They found that patients with mild or
minimal hepatitis C from liver biopsy were slower in tests of working memory. He
noted that although they were slow, their accuracy on these tasks was preserved,
and this has been described in chronic fatigue syndrome. There were no attention
or secondary memory abnormalities.
In the view of these
findings they asked themselves, if HCV infects cells in the CNS (central nervous
system), does this cause cerebral metabolite abnormalities, and is cerebral HCV
infection the cause of the observed neuropsychological symptoms? They carried
out a proton cerebral magnetic resonance spectroscopy study to determine if
metabolite abnormalities exist in the brain of patients with histologically mild
hepatitis C. They randomly selected 30 patients with biopsy proven mild or
minimal hepatitis due to HCV. As well, they studied 29 matched controls, and 12
eAG+ve patients with chronic HBV. No patient in the HBV or HCV groups had
significant fibrosis or cirrhosis. The researchers reported seeing metabolic
abnormalities in the testing in those with HCV compared to both normals
(volunteers) and chronic HBV patients. There were no statistical differences
between the normals and those with HBV. These abnormalities were not due to
hepatic encephalopathy. They described the abnormalities as being similar to
those abnormalities observed in HIV. Again, no patient in this study had
significant fibrosis or cirrhosis. None of the study participants had used IV
drugs in the 6 months preceding the study. There was no statistical difference
in the study results between those with or without prior drug use. Those with
prior drug use had the same abnormalities as those who never used IV drugs. The
researchers concluded that prior drug use did not affect the outcome of the
study.
Is
there direct infection by HCV of the CNS?
He presented a suggested potential model by which this could happen. Microglial
cells in the brain turn over slowly and are replenished by circulating monocytes,
possibly up to 30% in one year. Circulating monocytes are potentially infectable
by HCV, and may carry the virus across the blood brain barrier into the brain
and the microglial cells. Once in the cells they become activated and produce
chemokines, cytokines, and neurosteroids which may mediate the neuropsychiatric
symptoms described in this presentation. The question still remains--does HCV
infect the microglial cells in the brain? The only way to answer this question
is to conduct direct post mortem viralogic examination of brain tissue, which is
being currently undertaken at Imperial College School of Medicine in London.
He also suggested that
of equal or possibly greater importance is the possibility that the brain may
act as a sancutary site for HCV, allowing immune evasion and protection against
antiviral therapy. He suggested that cessation of viral production from the
liver may occur during phase 1 of viral decline after starting HCV therapy, but
the slower viral decline during phase 2 may be due to a continued release of
virus from the brain. He suggested that an alternative explanation for possible
brain dysfunction seen with HCV could be that systemic cytokines cross the
blood-brain barrier and may exert an effect. But he discounted this theory
because in this study patients with HBV had normal spectroscopy. HCV antiviral
therapy has been administered to the study patients and results are pending. In
the study reported at DDW, and discussed above, the study authors reported
therapy improved cerebral function, and they suggest their data may indicate a
direct action of HCV infection on the brain.
This report is comprised
mostly of studies presented in Durban (but also includes information from other
sources--journals, Retrovirus Conference) and details the prevalence of HCV in
HIV infected individuals with various backgrounds, exposure risks and
geographies-- from IVDUs to the potential for sexual transmission. Regarding the
risk of sexual transmission, HCV infection appears to occur more often in
persons with high risk sexual practices. Individuals with multiple sex partners
appear to be more at risk for HCV infection, while individuals in long-term
monogomous relationships appear to remain uninfected. These reports and previous
ones are piecing together a picture of the prevalence of coinfection. A number
of prevalence studies reported from various cities in the USA estimate that
60-90% of individuals with HIV due to IVDU has HCV as well. Based on these
studies it appears that there is a risk for contracting HCV from sexual contact.
The question remains --how much risk?
Barbara McGovern
reported at the 1999 November IDSA Conference on a look back at all HIV+
patients who died at her Boston based hospital from May '98 to April '99. She
found ESLD due to HBV/HCV was the leading cause of death in patients with
underlying HIV, even though 55% had undetectable HIV viral load and/or >300
CD4s. At Durban, Spinetti and an Italian research group reported increased
mortality due to ESLD in the post HAART era compared to the pre HAART era (12%
vs. 33%). Among the 308 in-hospital deaths occurring from 1987 to 1995, liver
failure was defined as the cause of death in 35 patients (12%). Among the 46
in-hospital deaths observed from 1998 to 1999, liver failure was defined as the
cause of death in 15 (33% p>0.01 vs. 1987-95). Multivariate analysis showed
that in-hospital liver related mortality was independently associated with
hepatitis B surface antigen reactivity (Odds Ratio, 9; 3.8-21.7), anti HCV
reactivity (OR 5,1.4-21), and history of alcohol abuse (OR: 2.3; 1-5.2).
There have been doubts
about that HCV could be transmitted sexually or that it could be transmitted
sexually at more than a very low rate. A recently published study found HCV in
breastmilk, and a second recently published study found HCV in male semen. A
Spanish research group reported breast milk HCV-RNA was negative in
nonviremic mothers and
positive in 20% of the viremic mothers. The rate of HCV transmission was higher
for infants of mothers with higher HCV viremia and also for infants whose
mothers were HCV-RNA-positive in breast milk. The authors said larger studies
are needed before advising avoidance of maternal breast feeding (Pediatr Infect
Dis J 2000 Jun;19(6):511-6: Ruiz-Extremera A et al).
Using a sensitive
testing method (PCR), a French research group reported eight seminal plasma
samples of 21 (38%) were found to contain HCV-RNA (6/8 were HIV+, 2/8 were
HIV-). HCV viral loads detected in semen were low, which suggests that the risk
of HCV sexual transmission is probably also low. Further studies using
experimental infection in a cell culture system or an animal model are needed to
prove that HCV-RNA positivity in semen reflects the presence of infectious virus
(Lancet 2000; 356: 42 - 43, Marianne Leruez-Ville et al).
An Italian study
reported recently that HCV was transmitted from mother to newborn 5% when HIV
was not present but 17% when the mother had HIV. At the Feb. 2000 HIV Retrovirus
Conference, Craib from British Vancouver reported on a study to determine HCV
prevalence and identify risk factors in a group of sexually active homosexual
men. In a random sample of 232 men, 120 were HIV+ (112 were HIV-). Of the 232
men 20 (8.6%) had HCV and HCV prevalence was significantly higher (6-fold) among
HIV+ than HIV- men (17/120 14% vs 3/112 2.7%). They reported the risk factors
for the HCV+ men. HCV+ men had more sexual partners in the past year (>= 20
partners: 80% vs 40%), and in their lifetime (>=100 partners: 90% vs 61%).
They also had greater incidence of receptive fisting (30% vs 12%; p=0.40),
insertive fisting (55% vs 25%; p=0.004), more often reported receptive oral-anal
contact (100% vs 85%; p=0.067), more often reported injection drug use (21% vs
2%; p<0.001), cocaine use (50% vs 24%; p=0.013), MDA use (70% vs 36%;
p=0.003), and amphetamine use (30% vs 13%; p=0.056). Multivariate analysis
showed injection drug use (p=0.024), being HIV+ (p=0.056), low education level
(p=0.031) and insertive fisting (p=0.032) to be independent risk factors for
being HCV+.
Zaltron and an Italian
research group reported on this study and suggest that people on HAART (n=82)
and treated with interferon due to elevated ALT can normalize ALT (33%), and
maintain viral load reductions. The study reports only 2 individuals had VL
increase of 0.5 log. IFN-a was given to people on HAART. Primary response was
defined as ALT normalization in the presence of undetectable HIV RNA at 3 &
6 months. Fifty seven patients (30 treated) completed the third month and 43 (24
treated) the sixth month of the study. PR was observed at three and six months
in 37% and 33% of treated patients and in none of untreated patients
(p>0.01). Two patients in the treated group showed HIVRNA increase greater
than 0.5 log copies/ml. Also seemingly important in this study is that CD4
count>500 and genotype 2 & 3 were associated with PR.
16%
HCV Sexual Exposure Prevalence in Spanish Study
In a study designed to
evaluate the prevalence, route of transmission and clinical significance that
current co-infection with TT virus (TTV), hepatitis C virus (HCV), and hepatitis
G virus (HGV) in HIV-1 infected patients, M Martinez from Barcelona, Spain
analyzed the presence of HCV in plasma samples from 160 infected patients with
parenteral (38 intravenous drug users 'IVDU's' and 41 patients with hemophilia)
or sexual (39 homosexuals and 42 heterosexuals) risk of exposure, and in 168
volunteer blood donors. Alanine aminotransferase (ALT) levels and CD4+ T cell
counts were also analyzed. Prevalences of HCV infection was higher among
patients with parenteral (needles by drug abuse) (62% and 68%) than in those
with sexual (17% and 16%) risk of exposure. But the study authors report 16%
risk of sexual transmission. Some of this 16% could be due to unidentified drug
use or an unwillingness to admit drug abuse.
To assess the impact of
HCV infection on clinical progression and on survival of HIV+ subjects in the
era of potent antiretroviral therapy (ART), G Greub of the ID Dept. at
University Hospital in Lausanne Switzerland looked at 2766 individuals followed
in the Swiss HIV Cohort Studies. They started potent ART between 01/01/95 and
03/31/99.
1011/2766
(36.6%) HIV+ subjects were HCV co-infected. 511/537 (95.2%) active drug user
were HCV+ as compared to 22.4% in
other groups.
HIV is associated
with sexual risk and HCV with injection risk among young injection drug users in
San FranciscoÍ312 Young IVDUsÍ29% had HBV, 45% had HCVÍ93% with HIV also
had HCV.
K.A. Page-Shafe from the
University of California San Francisco reported on this study examining HCV
prevalence and risk factors for HIV hepatitis B (HBV) and C (HCV) among young
injection drug users (YIDU) in San Francisco. YIDU (>30 years) recruited in 4
neighborhoods were questioned about injection and sexual behaviors, sources of
clean needles, knowledge and use of needle hygiene, history of STD and overdose
experience. Blood was drawn for HIV, HBV and HCV antibody testing.
312 YIDU participated,
193 (68%) males and 87 (31%) females. Median age was 22 (range: 15-29), and
median number of years injecting was 5 (range 0-19). Prevalence of HIV, HBV
(core antibody or surface antigen), and HCV was 6%, 29%, and 45%, respectively.
93% of those with HIV infection were co-infected with HCV or HBV. Variables
independently associated 'OR ;(95% CI)' with HCV seropositivity were: age (per 5
yr. increase) '2.2;(1.3-43.7)', years injecting (per 5 yr. increase)
'2.1;(1-3.5)', injected by a sex partner at initiation '3.9;(1.5-9.9)', ever
injected with someone else's used needle '2.5;(1.2-5.2)', bleached last time
injected with borrowed needle '0.5;(0.2-0.98)', snorted or smoked cocaine,
methamphetamine, or heroin in the prior year '0.4; (0.2-0.8)', injected daily
'4.4;(2.4-8.5)', and HBV '3.0;(1.5-6.0)'.
27/39
(69%) with HIV had HCV in Patients Presented in Emergency Room, Alberta, Canada
S. Houston at the
University of Alberta, in Edmonton, Alberta Canada reported on 3057 subjects
were entered in database. Subjects were younger, presented more frequently with
trauma and more often went on to admission than the general ED population (all
p>0.05). 71% presented with medical illnesses, 21% with trauma. 7%
self-identified as aboriginal. 37(1.2%, 95%CI = 1-2) were HIV-seropositive; 2
others demonstrated a banding pattern characteristic of acute seroconversion.
HIV infection was associated in multivariate analysis with aboriginal status,
age and HCV infection. 27 of all 39 (69%) HIV-infected subjects and both
seroconverters were HCV co-infected.
Histologic
improvements of liver despite virologic failure of interferon (IFN)+ribavirin
therapy in 3 HIV+/HCV+ patients
Shulman said in her
poster that although the usual outcomes measured for HCV treatment in studies
are HCV viral clearance and ALT normalization, follow up biopsy data in
treatment failures in HIV- cohorts show improvements in histology in over 30%.
Mitch Shiffman has published data (Gastroenterology 1999;117:1164-1172)
suggesting that maintenance therapy may be useful in maintaining improved
histology.
In an ongoing treatment
trial of IFN alpha, 3 million units TIW + ribavirin 800mg/d , 3 patients with
virologic failure at 6 months have received pre- and post-therapy liver
biopsies. Patients had post-treatment liver biopsies between 1-3 weeks after
discontinuing therapy.
All 3 (pt A, B, and C)
patients were males ages 44, 47, and 50 with baseline CD4 counts 234, 202, and
779. HCV genotypes were 1a, 3a, and 2b, and HCV RNA levels of 16,000,000/ml,
10,000,000/ml, and 250,000/ml. 2 patients had cirrhosis at baseline. None of the
3 had a substantial change in HCV RNA with monthly monitoring. ALT remained at
least 2X normal in all patients at all time points measured. Patient B did have
a 5-fold reduction in ALT from his baseline. The other two had no significant
reductions. Knodell scores improved in all three, 11 to 9, 16 to 13, and 15 to
8. Patient B had an apparent reduction in fibrosis as well.
Shulman concluded that, as has been shown in HIV- HCV+ patients, treatment of HCV with interferon-based therapy can lead to histologic benefits despite lack of HCV clearance or ALT normalization. Biopsy outcomes should be an important part of future therapeutic trials for these patients.