Nevirapine
in High Viral Loads (BI 1090 & Atlantic Study)
Reported
at Durban. BI 1090 was a prospective, double-blinded, placebo-controlled,
multi-center-clinical endpoint study in na‘ve and AZT experienced individuals
with <200 CD4s, receiving NVP+AZT/3TC or AZT/3TC. This is an analysis of only
the treatment-na‘ve individuals. Patients received nevirapine +AZT/3TC or
AZT/3TC. The Amplicor 1.5 Ultra-sensitive 50 copy Assay was used. The study was
conducted in South Africa, Argentina and USA/Europe, but about 80% of patients
were from South Africa, 58% were Black and 39% white in the NVP arm. Individuals
in the NVP arm had median CD4 count of 87, and viral load of 208,497 copies/ml
(5.3 log). Using the 50-copy assay, the median viral load reduction was about 3
log at about week 62 from baseline in the NVP arm. At 12 months, 42% (n=79) (ITT
analysis) had <50 copies/ml in the NVP arm (<0.01). The baseline viral
loads were divided into 4 groups based on the baseline values for the purpose of
analysis: <31,000, 31,000-104,000, 104,000 - 372,000, and >372,000
copies/ml. I don't think it's statistically significant, and there are about 20
patients in each of the 4 viral load groups, but there were no differences in
percent undetectable between <100,000 and >100,000 copies/ml. There was no
trend that people did worse in high viral loads. In fact in the 104,000-372,000
group, individuals did better than in the 31,000-104,000 group. The 4 CD4
quartiles were <40, 40-95, 95-159, and >159. But CD4s were important in
response. The two predictors for viral load failure were a person's baseline CD4
(<50 cells) and the viral load reduction at week 4 of study. Baseline viral
load was not predictive of response.
Study
participants in BI 1090 had more advanced HIV than those in Atlantic as measured
by CD4 & viral load. In the Atlantic Study, reported below, the number of
patients who had >58,519 copies/ml at baseline in the 48 week analysis
reported below was small, and the results were not statistically significant.
NVP and ddI were dosed once daily in Atlantic and twice daily in BI 1090. The
FDA recently recommended that twice daily ddI dosing was preferable to once
daily. It's possible that twice daily NVP produces better NVP blood levels than
once daily. The 3NN study is being planned to explore the NVP blood levels
question.
ATLANTIC
STUDY.
This study of treatment-na‘ve individuals compares nevirapine, indinavir, and a
triple NRTI regimen for safety, activity, and durability. Each of the 3 regimens
contain d4T+ddI combined with either 3TC, nevirapine, or indinavir. So, it is a
PI regimen vs a NNRTI nevirapine regimen vs. a triple 3TC-based NRTI
combination. Forty-eight week data was presented here in Durban, and it differs
from the 24-week data previously presented. This was the first study to compare
a triple nuke regimen, albeit a 3TC regimen, to PI and NNRTI regimens. The
48-week data shows the 3TC-based triple-nuke regimen, did worse, certainly
compared to the IDV arm. This result is more emphatic because the baseline
median viral loads were so low in all three treatment arms.
It
is an open-label, randomized, comparative study whose total duration is 144
weeks. The primary study objective was to assess the comparability of the 3
regimens investigated in reducing the serum HIV RNA load to undetectable levels.
298 patients were enrolled with asymptomatic HIV (CDC 1993 stage A). Standard
dosages of drugs were used except for NVP and ddI, which were dosed once daily.
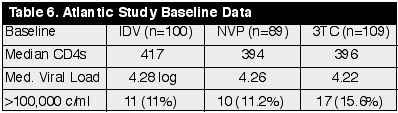
At
baseline, those receiving indinavir regimen (n=100) had median CD4 of 417 (range
329-560), viral load of 4.28 log (19,000 copies/ml), and 11 (11%) had
>100,000 copies/ml at baseline. In the NVP arm (n=89) at baseline, median CD4
was 394, viral load 4.26 log, and 10 (11.2%) had >100,000 copies/ml. In the
3TC arm (n=109), median CD4 was 396, viral load 4.25 log, and 17 (15.6%) had
>100,000 copies/ml. So, all three groups were relatively comparable in CD4s
and viral load. The number of patients with HIV-RNA >100,000 c/ml was small. (See
Table 6)

Results:
In
the ITT analysis after 48 weeks:
IDV-
49% <50 copies/ml (n=100)
NVP-
49% <50 copies/ml (n=89)
3TC-
40% <50 copies/ml (n=109)
Differences
between arms not statistically different: IDV vs NVP: p=0.95; IDV vs 3TC:
p=0.21; NVP vs 3TC: p=0.20.
On
Treatment (OT) Analysis:
IDV-
92% <50 copies/ml (n=54)
NVP-
82% <50 copies/ml (n=54)
3TC-
67%<50 copies/ml (n=66)
Only the IDV vs 3TC comparison was statistically significant, but the NVP arm vs 3TC was almost statistically significant. This shows that for people able to stay on the IDV regimen (OT analysis), in this study they did better than the 3TC based regimen. There was a trend towards statistical significance that individuals in the NVP arm did better than those in the 3TC arm. When comparing the IDV to the NVP arm, there was no statistical significance. (IDV vs NVP, p=0.16; IDV vs 3TC, p=0.002; NVP vs 3TC, p=0.068).
Higher
Viral Loads.
Among patients with HIV-RNA >58,519 copies/ml (upper quartile), more IDV
patients had HIV RNA <50 copies/ml at week 48 compared to NVP and 3TC, but
the analyses were not statistically significant. As well, the number of patients
in the analysis was small. (ITT: 48% [12/25], 28% [6/22], and 26% [7/27],
respectively, p=0.18; OT: 80%, 56%, 42%, respectively, p=0.08). Because the
p=0.08 was close to statistical significance, this suggests a trend that IDV was
superior in the OT analysis. But this probably only means a further study is
needed to answer this question. No difference was found with 500 HIV RNA c/ml as
the cutoff (OT: 80%, 78%, and 79%, respectively, p=0.99).
CD4s
increased across all 3 groups about 120-150 from baseline to week 48.
Clinical
Toxicities.
(excluding events unrelated to study drugs and events that occur in <5% of
subjects) (See Table 7)
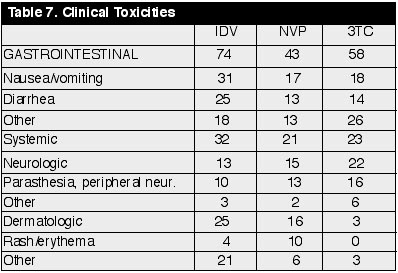
They did not list the incidence of elevated liver enzymes. The Atlantic 24-week data report at ICAAC 1999 had 55-68 individuals in the analysis of each of the 3 arms, and they reported the following incidence of liver enzyme elevations: 4 cases of grade 3 & 4 elevated LFTs in the IDV arm, 9 in NVP arm, and 5 in 3TC arm.