| |
|
The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: A long-term retrospective cohort study
|
| |
| |
Hepatology December 2001 o Volume 34 o Number 6
Vincent Di Martino et al
This study finds HCV progressed more quickly in HIV infected patients who had
never received HAART, only NRTI use. Not having received HAART is perhaps
important because the French have also reported a controversial finding that
HAART may slow HCV progression. They reported HAART with a PI may slow
progression. This is controversial because other studies have not found that
HAART has this beneficial effect. In some patients hepatoxicity from HAART
may accelerate progression. Certainly hepatoxicity (elevated ALT) occurs in
some patients on HAART but we do NOT know if this is clinically significant.
These questions have NOT been studied well. We need better answers to these
questions, so studies are needed. One study repored at both AASLD 2000 and
2001 (Kemm, abstract 611, 2001) found biopsies in patients on protease
therapy HAART appeared to have a "distinctive histologic pattern of liver
injury in biopsies from HIV patients that they did not see previously in this
population". They found this pattern in 26% (28/110) of patients on HAART.
There were limitations to the study. Biopsies before and after therapy were
not performed, only a single biopsy. Other potential contributing factors
could have been associated with results.
Di Martino also reported that interferon slowed progression to cirrhosis in
this study.
You can read a study by Di Martino, the same author, reported at the AASLD conference in November 2001 which follows coinfected patients with cirrhosis and their progression to more serious complications and death--
www.natap.org/2001/aasld2/day26.htm
Abstract-
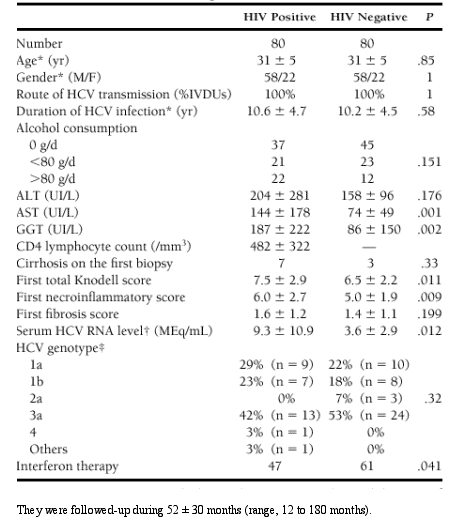
In this study we analyzed the influence of human immunodeficiency virus (HIV) infection on the course of chronic hepatitis C through multivariate analysis including age, alcohol consumption, immune status, and hepatitis C virus (HCV)-related virologic factors. Eighty HIV-positive and 80 HIV-negative injection drug users included between 1980 and 1995 were matched according to age, gender, and duration of HCV infection and followed-up during 52 months. The progression to cirrhosis was the primary outcome measure. The impact of HIV on HCV-RNA load, histologic activity
index, response to interferon therapy, and liver-related death was also considered.
In HIV-positive patients,
- chronic hepatitis C was characterized by higher serum HCV-RNA levels (P = .012), higher total Knodell score (P = .011), and poorer sustained response to interferon therapy (P = .009).
- High serum HCV-RNA level was associated with low CD4-lymphocyte count (P = .001).
- Necroinflamatory score was higher in HIV-positive patients (P = .023) independently of the CD4-lymphocyte count, whereas increased fibrosis was related to decreased CD4-lymphocyte count (P = .011).
- The progression to cirrhosis was accelerated in HIV-positive patients with low CD4 cell count (RR = 4.06, P = .024) and in interferon-untreated patients (RR = 4.76, P = .001), independently of age at HCV infection (P = .001).
- Cirrhosis caused death in 5 HIV-positive patients. The risk of death related to cirrhosis was increased in heavy drinkers (RR = 10.8, P = .001) and in HIV-positive patients with CD4 cell count less than 200/mm3 (RR = 11.9, P = .007).
- In this retrospective cohort study, HIV coinfection worsened the outcome of chronic hepatitis C, increasing both serum HCV-RNA level and liver damage and
decreasing sustained response to interferon therapy.
- Age and alcohol were cofactors associated with cirrhosis and mortality.
- Interferon therapy had a protective effect against HCV-related cirrhosis no matter what the patient's HIV status was.
(HEPATOLOGY 2001;34:1193-1199.)
The aims of this work were-
(1) to compare the virologic and histologic characteristics of chronic hepatitis C in HIV-positive and HIV-negative injection drug users matched with respect to age, gender, and duration of hepatitis C;
(2) to compare the progression to cirrhosis and the survival in both groups; and
(3) to evaluate through multivariate analyses the respective influence of HIV and HIV- related immunodepression on HCV viral level, histologic findings, progression to cirrhosis, and survival.
Study design
This was a retrospective cohort follow-up study conducted in H˘pital Beaujon including 80 patients with HIV-HCV coinfection and a past history of injection drug use who were consecutively admitted between 1980 and 1995 for a liver biopsy. As a control group, 80 HIV-negative patients with chronic hepatitis C and a past history of injection drug use who were admitted for a liver biopsy during the same period were included. These control patients were selected by individually matching with HIV-positive patients according to gender, age, and known duration of hepatitis C. In HIV-positive and HIV-negative patients, we compared the virologic characteristics of HCV infection, the biochemical and histologic activity of chronic hepatitis C, the progression to cirrhosis, and the survival rate. Studied patients had been seen for the first time between 1980 and 1995. The follow-up ended at death or in May 1997.
HIV-positive patients
Fifty-two patients received zidovudine, 21 before the first evaluation of the study and 31 during follow-up. Only 5 patients received didanosine and none received protease inhibitor, non-nucleoside analogue, or other nucleoside analogues of HIV reverse transcriptase during the follow-up period. A liver biopsy was systematically performed during the first 3 months after the first clinic. It was performed percutaneously in 75 patients, or, in 5 patients, by transjugular catheterism because of ascites, elevated prothrombin time, severe thrombopenia, or elevated bleeding time.
HIV-negative patients
One HIV-negative control patient was sought for each HIV-positive injection drug user. The controls were injection drug users followed for more than 12 months for chronic hepatitis C. They were randomly selected among a series of 243 consecutive patients seen during the same period by the same practitioner as the patient with HIV-HCV coinfection. The controls were individually matched to the HIV-positive patients with respect to age (within 5 years), gender, and the previous known duration of chronic hepatitis C (within 5 years), estimated by the delay between the onset of injection drug use and the first clinic in H˘pital Beaujon. The same criteria for inclusion and exclusion were used in selecting HIV-negative and HIV-positive patients. The same data were
recorded, except for the follow-up of HIV infection.
Interferon therapy
A single 6-month course of interferon alfa 2b therapy, 3 MU 3 times weekly, was given when the following conditions were present: (1) no contraindication for interferon therapy, (2) histologically proven chronic active hepatitis with a Knodell activity index of more than 6, (3) a CD4 lymphocyte count of more than 200/mm3 in HIV-positive patients, and (4) patient's consent. Forty-seven HIV-positive and 61 HIV-negative patients were thus treated (P = .041). Treated and untreated patients were similar with respect to age, gender, previous duration of chronic hepatitis C, serum alanine transaminase (ALT) level, and HCV genotype. Untreated patients were more often alcohol consumers (63% vs. 42%, P = .018), had higher serum HCV-RNA level (18.0 ▒ 11.7 vs. 3.8 ▒ 6.0 ? 106Eq/mL, P = .008) and lower Knodell score (6.3 ▒ 3.1 vs. 7.3 ▒ 2.4, P = .029) comparatively with treated patients. The CD4 cell count was not significantly
different in HIV-positive treated and HIV-positive untreated patients. The response to interferon therapy (biochemical response) was defined by the normalization of serum ALT at the end of therapy (end of treatment biochemical response) and at 6 months after the end of therapy (sustained biochemical response). Only biochemical responses to interferon therapy were analyzed, because numerous well-stored serum samples were missing for retrospective polymerase chain reaction analysis.
Virology
HCV genotype was retrospectively determined in 76 well-stored available serum samples by using a commercial probe hybridization assay (InnoLipa HCV; Innogenetics, Ghent, Belgium), which accurately identifies HCV genotypes 1 through 6, according to the Simmonds' classification.
Quantification of serum HCV RNA was performed with a commercial branched DNA signal amplification assay (Quantiplex HCV; Chiron, Emeryville, CA) from sera harvested before interferon therapy in treated patients and stored in optimal condition at -80░C. Only 62 sera (31 from HIV-positive patients, 31 from HIV-negative patients) were available for this analysis.
Baseline Characteristics of HIV Positive and HIV-Negative Patients
|
|
| |
 |
| |
Results
Progression to cirrhosis and survival
Progression to cirrhosis
At the first histologic evaluation, cirrhosis was found in 7 HIV-positive patients and in 3 HIV-negative patients (P = .32, Table 1 ). Baseline alcohol consumption (>80 g/d) was the only factor significantly associated with cirrhosis at baseline (P = .002), whereas liver biopsy did not evidence any sign of alcohol-induced hepatitis associated with cirrhosis. During follow-up, 10 additional patients (7 HIV positive and 3 HIV negative) developed cirrhosis, with a mean delay of 102 ▒ 47 months in HIV-negative patients and 64 ▒ 34 months in HIV-positive patients. All cases of cirrhosis were histologically proven after a
clinical or ultrasonographic suspicion.
--The prevalence of cirrhosis at the end of follow-up was 17.5% in HIV-positive patients and 7.5% in HIV-negative patients (P = .056).
--The actuarial rate of cirrhosis was significantly higher in HIV-positive
patients than in HIV-negative patients:
--7% vs. 3% at 10 years from HCV infection,
--37% vs. 10% at 20 years, and
--69% vs. 10% at 25 years (P = .040, Fig. 1).
No relationship was found between progression to cirrhosis and continued alcohol consumption. In interferon-treated patients without cirrhosis at baseline (n = 103), an end-of-treatment biochemical response to interferon was associated with a lower rate of subsequent cirrhosis (P = .003). Conversely, neither gender nor self-reported alcohol consumption at baseline had significant influence on the progression to cirrhosis (Table 2).
Multivariate analysis identified 3 independent predictors of subsequent cirrhosis:
--HIV coinfection with low CD4 cell count during follow-up (relative risk [RR] =
4.06),
--old age at HCV infection, and
--absence of interferon therapy (RR = 4.76) (Table 2 ).
Survival
Death occurred in 10 HIV-positive and 3 HIV-negative patients. HCV-related cirrhosis was present at the time of death in 8 HIV-positive and 3 HIV-negative patients. Five HIV-positive patients died of HCV-related cirrhosis. One HIV-infected patient, who kept a CD4 cell count of more than 400/mm3 during the whole follow-up, died from hepatocellular carcinoma. The others died from variceal bleeding (n = 3) and spontaneous peritonitis (n = 1). Death was related to AIDS in 5 HIV-positive patients (including 2 cirrhotic patients). The actuarial rate of death related to cirrhosis was higher in HIV-positive patients with a CD4 cell count of less than 200/mm3 (RR = 11.91, P = .007), in heavy drinkers (RR = 10.78, P = .001), and was positively correlated to age (P = .026). Patients who received interferon therapy had a lower risk of death related to cirrhosis (RR = 0.20, P = .031), (Table 3).
|
|
| |
|
|
|