AASLD Report On HCV Therapy Toxicities
HEMOGLOBIN DECREASES FROM COMBINATION HCV THERAPY INCLUDING RIBIVARIN
BRIEF SUMMARY of this first study:
1600 patients receiving daily or 3 times per week IFN + RBV (1000/1200 mg/day) were analyzed
all patients had >12 or 13 g/dL hemoglobin (Hb) before HCV therapy
As expected, 10% (57/551) had <10 g/dl following initiation of HCV therapy
Incidence of <10 g/dL Hb 5 times greater in women than men (20% vs 4.8%)
Men appeared at greater risk to have >3 g/dL Hb decrease than women
Daily IFN dosing did not have greater decrease in Hb than three times per week IFN dosing suggesting than pegylated IFN with its higher blood levels should not result in greater Hb decreases
Second and third studies below report the affect of once-weekly epoetin alfa (recombinant human erythropoietin) (EPO) treatment for RBV-associated anemia.
Here is a BRIEF SUMMARY:
Study 2:
All
HCV/HIV coinfected patients with Hgb (hemoglobin) <10g/dL were treated with
40,000 units of recombinant human erythropoietin for at least 3 months. After
about 4 weeks Hb increased to 12.7 g/dL in 4/5 patients in the IFN+RBV group and
after 8 weeks in the remaining patient. 1 patient discontinued the study due to
intractable anemia. The 1 patient in the IFN alone group responded to
erythropoietin after 8 weeks of treatment
Study 1:
mean lower than normal 11.2 g/dL Hb during
therapy increased to 13.9 g/dL in the patients receiving EPO. In the group not
receiving EPO Hb did not improve
CHANGES IN HEMOGLOBIN DURING THERAPY WITH INTERFERON ALFA-2B PLUS
RIBAVIRIN IN IFN-NAÔVE AND EXPERIENCED PATIENTS
Mark S Sulkowski, Johns Hopkins Univ,
Baltimore, MD; Ronald Wasserman, Linda J Brooks, CA Pacific Medical Ctr, San
Francisco, CA; Lisa Ball, Hepatitis Resource Network, Tacoma, WA; Robert G Gish,
CA Pacific Medical Ctr, San Francisco, CA
Background
Combination therapy with
ribavirin is associated with a dose-dependent, reversible hemolytic anemia in
many patients, resulting in a mean hemoglobin decrease of approx. 2.5 g/dL and a
hemoglobin < 10 g/dL in approx. 10% patients. However, other factors
associated with significant anemia have not been well described and it is
unknown if more frequent administration of IFN (e.g. daily or pegylated) will
result in greater anemia due to impaired compensatory reticulocytosis. (what
about maintenance therapy with IFN or IFN+RBV, is Hg a greater concern in this
circumstance?)
Methods:
We retrospectively analyzed
treatment-related changes in hemoglobin level in participants of two interferon
alfa-2b plus ribavirin treatment studies. This 655 patient database included:
Study 1, 170 IFN-naÔve pts randomized to receive RBV 1000 - 1200 g/d (based on
wt) plus IFN alfa-2b 3 MIU daily or TIW for 48wks and Study 2, 485 IFN-experienced
pts randomized to receive RBV 1000 g/d plus IFN alfa-2b 3 MIU daily or TIW for 4
wks followed by TIW dosing. Per protocol, all pts had Hb level > 12 g/dL
female or > 13 g/dL male patients at the time of enrollment. Pre-treatment
clinical and demographic data included gender, age, ethnicity, weight,
creatinine, ALT, CBC and liver histology. Hb values were obtained at treatment
wks 0, 1, 2, 4, then monthly to wk 48 and at post-treatment wks 4, 8,12, 24 and
48 (study 2).
Results:
As expected, 10.3% (57/551) had a
Hb < 10g/dL (95% CI 7.9-13.2). However, the incidence of anemia (Hb < 10g/dL)
was approx. 5-fold greater in women (20%, 95% CI 13.7 -27.5) than men (4.8%, 95%
CI 2.9 -7.5%) [RR 4.6, 95% CI 2.73 - 7.8]. While Hb decrease > 3 g/dL was
observed in 56% (308/551) of all pts, the incidence was greater in men (61%)
than women (45%) [RR 1.4, 95% CI 1.1- 1.6]. In addition, greater decreases in Hb
were observed in those subjects with higher baseline Hb levels (P < .000)
(See table). Compared to standard TIW dosing, daily IFN therapy was not
associated with a greater decline in Hb by treatment wk 4 in study 1 (- 2.6 g/dL
QD vs. 2.6 g/dL TIW, P = .98) or study 2 (- 2.2 g/dL QD vs, 2.0 g/dL TIW, P =
.28) nor at any additional timepoint in study 1. Further multivariate regression
analysis will be performed.
Conclusions:
While women were 5-times more
likely to experience a Hb < 10 g/dL during combination therapy, men were at
greater risk to experience a greater than 3 g/dL decline from baseline and
higher baseline Hb levels were associated with greater decrease in Hb. Of
significance, daily IFN use did not impact the Hb decrease, suggesting
PEG-interferon will not significantly affect RBV-associated anemia. Further
research is needed to evaluate strategies to minimize treatment-associated
anemia. This research was supported by unrestricted grants from Schering-Plough
and Ortho-Biotec, Inc.

ONCE WEEKLY EPOETIN ALFA INCREASES HEMOGLOBIN AND DECREASES RBV
DISCONTINUATION AMONG HCV PATIENTS WHO DEVELOP ANEMIA ON RBV/INF THERAPY
Ronald Wasserman, Infectious Disease Specialists Medical Group, Walnut
Creek, CA; Norbert Brau, Bronx VA Medical Ctr, Bronx, NY; Tarek I Hassanein,
Univ of CA, San Diego, CA; Edmund Bini, New York VA Med Ctr, New York, NY; Mark
Sulkowski, Johns Hopkins U Sch of Medicine, Baltimore, MD; D Dieterich, Liberty
Medical, LLP, New York, NY
Background:
Anemia frequently complicates ribavirin/interferon
(RBV/IFN) treatment (tx) for hepatitis C (HCV) and is often managed with RBV
dose reduction or discontinuation (d/c). RBV antiviral activity warrants optimal
dosage and its reduction or d/c negatively impacts HCV tx. A recent study
reported increased hemoglobin (Hb) following epoetin alfa (EPO) treatment for
RBV-associated anemia but did not report RBV dose changes or d/c's.1
This study further evaluates the safety and efficacy of EPO for RBV-associated
anemia and examines its impact on RBV dosing.
Methods:
Open-label, randomized, multicenter study of 60 HCV
pts with Hb £12 g/dL during the first 24 weeks (wks) of RBV/IFN tx. Pts receive
EPO 40,000 units s.c. wkly for up to 36 wks, or standard of care (SOC) including
RBV dose reduction, but not EPO. Primary efficacy endpoint is Hb change at 16
wks. Secondary endpoints include RBV dose change or d/c.
Results:
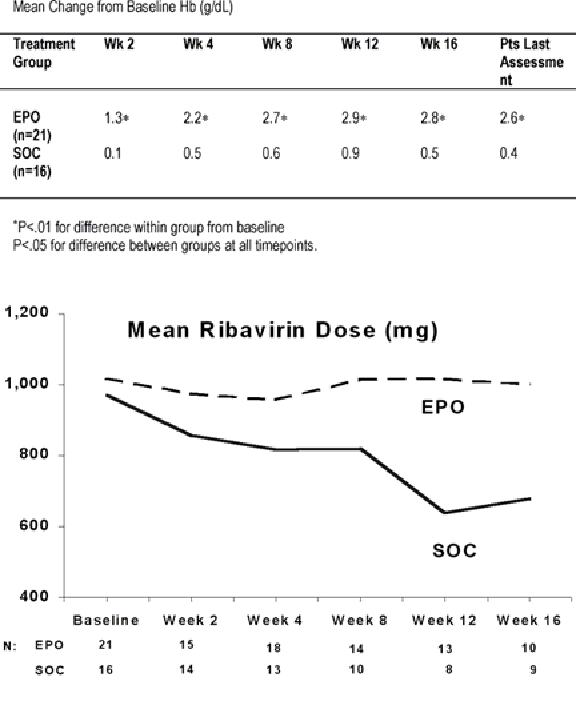
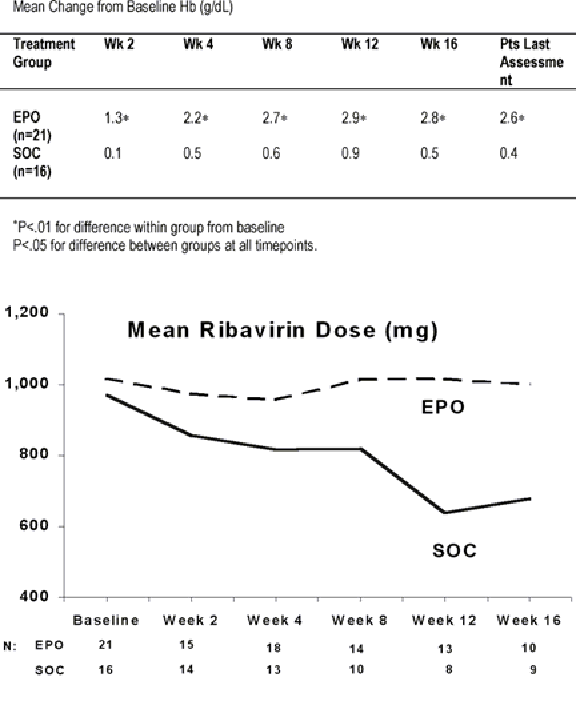
Thirty-seven pts have enrolled. Groups are similar at
baseline; mean age 49 years, 76% males, mean Hb 11.2 g/dL. At follow-up, Hb
increases in EPO group pts were significantly > baseline (P<.01) and SOC
group Hb increases (P<.05) Last recorded mean Hb was 13.9 g/dL in the EPO
group vs 11.6 g/dL in the SOC group (P<.05) By Wk 16 mean RBV dose decreased
17 mgs and 291 mgs from baseline in the EPO and SOC groups, respectively. At wk
16, RBV dose was reduced from baseline in 3 of 9 SOC group pts; doses remained
at baseline or were increased in 10 of 10 EPO group pts.
Conclusion:
Once weekly EPO is well-tolerated,
significantly increases Hb in anemic pts receiving RBV/IFN and may allow
continuation of optimal RBV doses. The study is ongoing. 1Weiss, KB
et al. Erythropoietin Use for RBV/IFN-Induced Anemia in HCV Patients [abstract] ICAAC 1998.
Treatment of HCV with
Interferon+Ribivarin in HCV/HIV Co-Infection
This was reported at the 2000 Human
Retrovirus Conference
D. Dieterich, K. Weisz, D. Goldman and others reported a 12 month follow-up from their small study (mean age 40 yrs) of treating HCV/HIV co-infection with interferon 3 MIU 3X/week (n=10) or combination interferon+ribivarin (n=11). The IFN alone group crossed over after 3 months to IFN+RBV. 19/21 were receiving HAART with either AZT or d4T. Although they could observe the effect of the in vitro finding that RBV inhibits phosphorylation of AZT and d4T, they did not actually study the effect of phosphorylation by ribivarin on AZT and d4T. Median liver biopsy score was 1.9 for both groups. After 3 months, HCV-RNA (viral load) for individuals receiving IFN alone did not decrease, but their CD4s remained the same and HIV-RNA decreased from 1500 to <400 copies/ml. While at 3 months for those receiving IFN+RBV, their HCV-RNA decreased from 325,000 to 600 copies/ml (4/8 had undetectable HCV viral load). Their absolute CD4 counts declined from 544 to 237, and their median HIV viral load remained <400 copies/ml. It is thought that absolute count doesn't matter if CD4 percent remains the same and in this study the percent remained about the same. After a while on IFN+RBV therapy, the absolute count may rebound back to where it was prior to HBV+RBV therapy. After 3 months all patients receiving IFN alone added RBV.
At 12 months, the median HCV viral load was undetectable for the group receiving IFN+RBV from the beginning. Their CD4 count was 311, and HIV viral load was <400 in 4/4 patients (I said the study was small). After irritability, the main adverse event was anemia. It occurred more often in the RBV+IFN group (5/11) than for those receiving IFN alone (1/10). But apparently, the anemia can be successfully treated with erythropoietin (r-HuEPO). All patients with Hgb (hemoglobin) <10g/dL were treated with 40,000 units of recombinant human erythropoietin for at least 3 months. After about 4 weeks Hgb increased to 12.7 g/dL in 4/5 patients in the IFN+RBV group and after 8 weeks in the remaining patient. 1 patient discontinued the study due to intractable anemia. The 1 patient in the IFN alone group responded to erythropoietin after 8 weeks of treatment.
Since 19/21 patients were taking AZT or d4T and all in the RBV+IFN group reduced HCV viral load from a median 325,000 copies/ml at baseline to undetectable, this suggests that the potential interaction previously seen in vitro between RBV and d4T and AZT may not be a problem. But further study is needed.
SIGNIFICANT RAPID SUPPRESSION OF HEMATOPOIESIS BY A SINGLE DOSE OF IFN-![]() 2B
2B
Martina Wichlas, Monika Homoncik, Anna
Kreil, Harald Hofer, Wolfgang Jessner, Alfred Gangl, Peter Ferenci, Markus Peck-Radosavljevic,
Univ of Vienna Medical Sch, Vienna Austria
Treatment with interferon-![]() (IFN-
(IFN-![]() )
induces profound hematologic alterations, mainly through IFN-induced suppression
of hematopoiesis in the bone marrow. This effects occur early in the course of
treatment, usually within the first month of therapy.
)
induces profound hematologic alterations, mainly through IFN-induced suppression
of hematopoiesis in the bone marrow. This effects occur early in the course of
treatment, usually within the first month of therapy.
AIM: To evaluate the acute effects of a
single dose of IFN-![]() on the erythroid, myeloid, and megakaryocytic lineages in
patients with chronic hepatitis C.
on the erythroid, myeloid, and megakaryocytic lineages in
patients with chronic hepatitis C.
METHODS:
18 IFN-naive patients with
chronic hepatitis C (subtype 1a or 1b) received a single dose of 10 MU IFN-![]() 2b (IntronA1,
AESCA-Schering Plough, Traiskirchen, Austria). Complete blood counts (CBC),
hematopoietic growth factor levels (erythropoietin EPO, thrombopoietin TPO), þ-thromboglobulin
as marker of platelet activation, and markers of bone marrow production of
platelets (reticulated platelets: RP's) and erythrocytes (reticulocytes: RC's)
were obtained prior to and 1 day after IFN-
2b (IntronA1,
AESCA-Schering Plough, Traiskirchen, Austria). Complete blood counts (CBC),
hematopoietic growth factor levels (erythropoietin EPO, thrombopoietin TPO), þ-thromboglobulin
as marker of platelet activation, and markers of bone marrow production of
platelets (reticulated platelets: RP's) and erythrocytes (reticulocytes: RC's)
were obtained prior to and 1 day after IFN-![]() 2b administration.
2b administration.
RESULTS:
IFN-![]() 2b caused an immediate
decrease of hemoglobin HB (median: 3.9%; Wilcoxon matched pairs test WT:
p=0.002), white blood count WBC (median: 19.6%; p=0.005), and peripheral
platelet count PPC (median: 14.9%; p=0.0005) within 1 day of IFN-administration.
These changes were reversible within 7 days without IFN-therapy. No hemolysis
was observed and no platelet activation and consumption could be detected as
evidenced by lack of increase of þ-thromboglobulin levels in plasma. The
decrease in CBC was neither accompanied by an increase in hematopoietic growth
factors in serum (EPO: p=0.9, TPO: p=0.12) nor by a compensatory increase in
bone marrow production of erythrocytes or platelets. Reticulated platelets as
precursor cells of platelets remained unaffected by the decrease in PPC
(p=0.08), while reticulocyte-production even decreased significantly within one
day of IFN-treatment by a median of 17.6% (p=0.003).
2b caused an immediate
decrease of hemoglobin HB (median: 3.9%; Wilcoxon matched pairs test WT:
p=0.002), white blood count WBC (median: 19.6%; p=0.005), and peripheral
platelet count PPC (median: 14.9%; p=0.0005) within 1 day of IFN-administration.
These changes were reversible within 7 days without IFN-therapy. No hemolysis
was observed and no platelet activation and consumption could be detected as
evidenced by lack of increase of þ-thromboglobulin levels in plasma. The
decrease in CBC was neither accompanied by an increase in hematopoietic growth
factors in serum (EPO: p=0.9, TPO: p=0.12) nor by a compensatory increase in
bone marrow production of erythrocytes or platelets. Reticulated platelets as
precursor cells of platelets remained unaffected by the decrease in PPC
(p=0.08), while reticulocyte-production even decreased significantly within one
day of IFN-treatment by a median of 17.6% (p=0.003).
CONCLUSION:
A single dose of IFN-![]() already
causes a significant decrease in the corpuscular elements of the blood. This
significant drop seems to be mainly due to IFN-induced myelosuppression as
demonstrated in in-vitro experiments.
This is evidenced in our study by the complete lack of increase of bone marrow
production of precursor cells of mature red cells and platelets. Interestingly,
no compensatory increase in hematopoietic growth factors could be demonstrated,
as would be expected in states of bone marrow suppression. For TPO, this could
be explained by downregulation of TPO production, which has been described in in-vitro studies (Peck-Radosavljevic;
Hepatology 1998; 28: 1424) and seems to occur posttranscriptionally. The exact
mechanisms for lack of EPO increase in this setting have not been elucidated so
far, but posttranscriptional suppression of EPO-production has been described
for other drugs like ciclosporin and needs further investigation during IFN-treatment.
already
causes a significant decrease in the corpuscular elements of the blood. This
significant drop seems to be mainly due to IFN-induced myelosuppression as
demonstrated in in-vitro experiments.
This is evidenced in our study by the complete lack of increase of bone marrow
production of precursor cells of mature red cells and platelets. Interestingly,
no compensatory increase in hematopoietic growth factors could be demonstrated,
as would be expected in states of bone marrow suppression. For TPO, this could
be explained by downregulation of TPO production, which has been described in in-vitro studies (Peck-Radosavljevic;
Hepatology 1998; 28: 1424) and seems to occur posttranscriptionally. The exact
mechanisms for lack of EPO increase in this setting have not been elucidated so
far, but posttranscriptional suppression of EPO-production has been described
for other drugs like ciclosporin and needs further investigation during IFN-treatment.
MULTIPLE DAILY DOSES OF IFN-![]() 2B CAUSE PROFOUND SUPPRESSION OF
HEMATOPOIESIS WITHOUT ADEQUATE BONE MARROW REGENERATION DESPITE A COMPENSATORY
INCREASE IN HEMATOPOIETIC GROWTH FACTORS
2B CAUSE PROFOUND SUPPRESSION OF
HEMATOPOIESIS WITHOUT ADEQUATE BONE MARROW REGENERATION DESPITE A COMPENSATORY
INCREASE IN HEMATOPOIETIC GROWTH FACTORS
Monika Homoncik, Anna Kreil, Martina
Wichlas, Harald Hofer, Wolfgang Jessner, Alfred Gangl, Peter Ferenci, Markus
Peck-Radosavljevic, Univ of Vienna Medical Sch, Vienna Austria
Treatment with interferon-a(IFN-![]() ) induces
profound hematologic alterations, mainly through IFN-induced suppression of
hematopoiesis in the bone marrow. This effects occur early in the course of
treatment, usually within the first month of therapy. No data on the early
hematologic response regarding growth factors and markers of bone marrow
regeneration after daily administration of IFN-
) induces
profound hematologic alterations, mainly through IFN-induced suppression of
hematopoiesis in the bone marrow. This effects occur early in the course of
treatment, usually within the first month of therapy. No data on the early
hematologic response regarding growth factors and markers of bone marrow
regeneration after daily administration of IFN-![]() are available to date.
are available to date.
AIM
To evalute the early effects of daily
doses of IFN-![]() on the erythroid, myeloid, and megakaryocytic lineages in
patients with chronic hepatitis C.
on the erythroid, myeloid, and megakaryocytic lineages in
patients with chronic hepatitis C.
METHODS
17 patients with chronic hepatitis
C (subtype 1a or 1b) received daily doses of 5 MU IFN-![]() 2b monotherapy (IntronA1,
AESCA-Schering Plough, Traiskirchen, Austria) for 14 days. Complete blood counts
(CBC), hematopoietic growth factor levels (erythropoietin EPO, thrombopoietin
TPO), þ-thromboglobulin as marker of platelet activation, and markers of bone
marrow production of platelets (reticulated platelets: RP's) and erythrocytes (reticulocytes:
RC's) were obtained at 4 timepoints prior to and during IFN-
2b monotherapy (IntronA1,
AESCA-Schering Plough, Traiskirchen, Austria) for 14 days. Complete blood counts
(CBC), hematopoietic growth factor levels (erythropoietin EPO, thrombopoietin
TPO), þ-thromboglobulin as marker of platelet activation, and markers of bone
marrow production of platelets (reticulated platelets: RP's) and erythrocytes (reticulocytes:
RC's) were obtained at 4 timepoints prior to and during IFN-![]() 2b administration.
2b administration.
RESULTS:
IFN-![]() 2b caused an immediate decrease of hemoglobin HB (median: 5.5%;
Wilcoxon matched pairs test WT: p=0.0006), white blood count WBC (median: 40.3%;
p=0.0003), and peripheral platelet count PPC (median: 26.6%; p=0.001) during the
14-day course of IFN-administration. No hemolysis was observed and no
platelet activation and consumption could be detected as evidenced by lack of
increase of þ-thromboglobulin levels in plasma. Contrary to observations after a
single dose of IFN-
2b caused an immediate decrease of hemoglobin HB (median: 5.5%;
Wilcoxon matched pairs test WT: p=0.0006), white blood count WBC (median: 40.3%;
p=0.0003), and peripheral platelet count PPC (median: 26.6%; p=0.001) during the
14-day course of IFN-administration. No hemolysis was observed and no
platelet activation and consumption could be detected as evidenced by lack of
increase of þ-thromboglobulin levels in plasma. Contrary to observations after a
single dose of IFN-![]() 2b, the decrease in CBC after multiple doses of IFN-
2b, the decrease in CBC after multiple doses of IFN-![]() was
accompanied by an increase in hematopoietic growth factors in serum (EPO mean
increase: 250%; p=0.008, TPO mean increase: 44.5%; p=0.007) which was
nevertheless insufficient to induce significant bone marrow production of
platelets (PR's pre vs. during IFN-treatment:
p=0.31). The increase in EPO-levels was even insufficient to prevent a drop in
bone marrow production of erythrocytes (RC's median drop: 21.5%; p=0.02).
was
accompanied by an increase in hematopoietic growth factors in serum (EPO mean
increase: 250%; p=0.008, TPO mean increase: 44.5%; p=0.007) which was
nevertheless insufficient to induce significant bone marrow production of
platelets (PR's pre vs. during IFN-treatment:
p=0.31). The increase in EPO-levels was even insufficient to prevent a drop in
bone marrow production of erythrocytes (RC's median drop: 21.5%; p=0.02).
CONCLUSION:
Multiple doses of IFN-![]() a
already cause a significant decrease in the corpuscular elements of the blood.
This significant drop seems to be mainly due to IFN-induced myelosuppression as
demonstrated in in-vitro experiments
and evidenced in our study by the complete lack of increase of bone marrow
production of precursor cells of mature red cells and platelets. The decrease in
platelets and red cells after multiple doses of IFN causes a compensatory
increase in hematopoietic growth factors, as would be expected in states of bone
marrow suppression. This increase is insufficient to overcome the bone marrow
suppression induced by IFN-
a
already cause a significant decrease in the corpuscular elements of the blood.
This significant drop seems to be mainly due to IFN-induced myelosuppression as
demonstrated in in-vitro experiments
and evidenced in our study by the complete lack of increase of bone marrow
production of precursor cells of mature red cells and platelets. The decrease in
platelets and red cells after multiple doses of IFN causes a compensatory
increase in hematopoietic growth factors, as would be expected in states of bone
marrow suppression. This increase is insufficient to overcome the bone marrow
suppression induced by IFN-![]() . For TPO, this could be explained by downregulation
of TPO production, which is associated with an inappropriately low TPO-increase
during IFN-treatment. This has been observed previously in-vivo and in-vitro and seems to be due to a posttranscriptional block in TPO-production
(Peck-Radosavljevic; Hepatology 1998; 28: 1424). Mean increase of EPO serum
levels by 250% seem to indicate an adequate EPO-response with regards to a 5.5%
drop in hemoglobin-levels, which nevertheless could not reverse the block in
bone marrow maturation/release of reticulocytes.
. For TPO, this could be explained by downregulation
of TPO production, which is associated with an inappropriately low TPO-increase
during IFN-treatment. This has been observed previously in-vivo and in-vitro and seems to be due to a posttranscriptional block in TPO-production
(Peck-Radosavljevic; Hepatology 1998; 28: 1424). Mean increase of EPO serum
levels by 250% seem to indicate an adequate EPO-response with regards to a 5.5%
drop in hemoglobin-levels, which nevertheless could not reverse the block in
bone marrow maturation/release of reticulocytes.