|
NATAP REPORTS |
Current Review & Update on Hepatitis C & HIV/HCV Cection |
SUMMER 2001 |
|
Adverse Events, Safety, Tolerability and Quality of Life
|
|
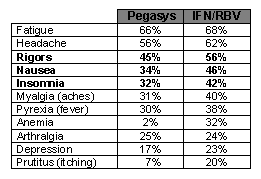
Following is a review of results from studies so far conducted. Still remaining to be seen is more widespread and long-term experience and observation from clinical use by doctors and patients. The rate of withdrawal due to symptomatic adverse events was 9.4% for standard IFN/RBV and 6.9% for Pegasys/RBV. Withdrawal due to a laboratory abnormality was 0.9% for IFN/RBV vs 2.6% for Pegasys/RBV. Neutropenia was the most common laboratory abnormality leading to withdrawal, which occurred in 3 patients. Neutropenia is a reduction in neutrophils, which are an important type of white blood cell. At the 2000 November AASLD, results were reported from the PegIntron/RBV study on safety and adverse events by John McHutchison. However, the presentation was limited to those patients in arms 2 and 3 whose baseline body weights led to ribavirin concentrations that were greater than 10.6 mg per kilogram. For the patients in the upper concentration range of ribavirin (greater than 10.6 mg/kg), adverse events that occurred with a rate that was at least 10% higher in the peginterferon 1.5 µg/kg arm (PEG) than in the standard interferon arm (IFN) were as follows: fever (46% in PEG versus 33% in IFN), nausea (43% PEG vs. 33% IFN); weight loss (30% vs. 20%); hair loss (45% vs. 32%); weakness (asthenia, 28% vs. 18%); and skin reaction at injection site (58% vs. 36%). Other adverse events that occurred in at least 10% of both treatment arms included: malaise, fatigue, headache, chills, "flu"-like symptoms, sweating, loss of appetite, diarrhea, vomiting, muscle and bone pains, joint aches, muscle aches (myalgias), anxiety, concentration ("thinking") problems, depression, insomnia (difficulty sleeping), irritability, coughing, shortness of breath, itchy skin, rash, and dry skin. The following additional adverse events profiles have been reported. Nearly all patients experience one or more adverse events. In many but not all cases, adverse events resolved after dose reduction or discontinuation of therapy. Some patients experienced ongoing or new serious adverse events during the 6-month follow-up period. In the PegIntron monotherapy study comparing PegIntron 1.5 ug/kg (n=304) to Intron A 3 MIU 3x/week (standard IFN given 3 times per week; n=307), some key adverse events rates reported were: headache (64% vs 58%), fatigue (45% vs 50%), flu-like symptoms (25% vs 19%), rigors (43% vs 33%), fever (40% vs 30%), nausea (25% vs 20%), diarrhea (20% vs 16%), abdominal pain (13% vs 11%), neutropenia (6% vs 2%), thrombocytopenia -reduced platelet counts- (7% vs <1%), myalgia- (61% vs 53%), arthralgia (31% vs 27%), anxiety/irritability/emotional lability (28% vs 34%), alopecia -hair loss- (22% vs 22%), dry skin (11% vs 9%), rash (6% vs 7%), depression (27% vs 25%), insomnia (20% vs 23%), hypothyroidism (5% vs 3%), weight decrease (21% vs 13%), injection site inflammation (40% vs 16%). In the study comparing PegIntron 1.5 ug/kg+RBV (n=511) to Intron A/RBV (n=505), common adverse events reported in the PEG-Intron/RBV group included myalgia (56%), arthralgia (34%) nausea (43%), anorexia (32%), weight loss (29%), alopecia (36%), and pruritus--severe itching- (29%). In the PEG- Intron/RBV combination therapy trial the incidence of severe adverse events was 23% in the INTRON A/RBV group and 31-34% in the PEG-Intron/RBV groups. The incidence of life-threatening adverse events was < 1% across all groups in the monotherapy and combination therapy trials. The most common adverse events were psychiatric which occurred among 77% of patients and included most commonly depression, irritability, and insomnia, each reported by approximately 30-40% of subjects in all treatment groups. Suicidal behavior (ideation, attempts, and suicides) occurred in 2% of all patients during treatment or during follow-up after treatment cessation. Incidence rates for other selected common adverse events comparing the PegIntron/RBV arm with the Intron A/RBV arm included: fatigue (66% vs 63%); weight decrease (29% vs 20%); concentration impaired (17% vs 21%); agitation (8% vs 5%); rash (24% vs 23%); dry skin (24% vs 23%). By the end of the 6-month follow-up period the incidence of ongoing adverse events by body class in the PEG-INTRON 1.5/RBV group was 33% (psychiatric—irritability, insomnia, anxiety, etc), 20% (musculoskeletal--myalgia, arthralgia), and 10% (for endocrine and for GI). In approximately 10-15% of patients weight loss, fatigue and headache had not resolved. Severe potentially life- threatening neutropenia (<0.5 x 10 9 /L) occurred in 1% of patients treated with PEG-Intron monotherapy, 2% of patients treated with INTRON A/RBV and in 4% of patients treated with PEG-Intron/RBV. Intron A is the brand name for standard interferon from Schering. Two percent of patients receiving PEG-Intron monotherapy and 18% of patients receiving PEG-Intron /RBVrequired modification of interferon dosage. Few patients (< 1%) required permanent discontinuation of treatment. Decreases in neutrophil counts were seen in 85% receiving combination PegIntron/RBV and in 60% receiving standard IFN/RBV. Severe depression in platelet counts (<50,000) occurred in 1% of patients taking PegIntron. The incidence and severity of neutropenia & thrombocytopenia were greater in the PegIntron group compared to the Intron A group. Neutrophil and platelet counts were decreased in 70% and 20% of patients (compared to 6% for those receiving Intron A/RBV), respectively, but generally return to pretreatment levels within 4 weeks after stopping therapy. Patients may require discontinuation or dose modification as a result of platelet decreases. In the PEG-Intron/RBVcombination therapy trial 1% or 3% of patients required dose modification of INTRON A or PEG-Intron respectively. Platelet counts generally returned to pretreatment levels within 4 weeks of the cessation of therapy. In an analysis that included only patients in the upper ribavirin concentration range, study discontinuation due to adverse events occurred among 14% of PegIntron/RBV patients and among 13% of Intron A/RBV patients. The total discontinuation rates due to any reason were not presented. The discontinuation rate was 20% in the registrational IFN/RBV study, and its been suggested that the reduced discontinuation rates in the PegIntron/RBV study is due to better patient/side effect management. Dose modification was 34% in IFN/RBV arm and 49% in the Peg/RBV arm compared to 10-25% in the registrational 48 week IFN/RBV study. Discontinuation for anemia was 0.8% in the full dose 1.5 ug/kg PegIntron/RBV arm compared to 0.2% in the IFN/RBV arm. Anemia (decrease of hemoglobin to less than 10 grams per deciliter) occurred among 14% of those taking PEG 1.5 mg/kg compared to 12% of those taking IFN. However, dose reduction due to anemia took place among the opposite percentages: 12% of those taking PEG, compared to 14% of those taking IFN. Yet, discontinuation due to anemia occurred only among 2% of PEG patients and 0.2% of IFN patients. Anemia associated with ribavirin may result in a worsening of cardiac disease. High-fat meals increase ribavirin blood levels. Neutropenia occurred more often in the full dose 1.5 mg/kg Peg/RBV arm (grade 3- 18% vs 7%; grade 4- 2% vs 4%). Neutropenia (low white cell count) led to a dose reduction in 21% of PEG patients, but in only 8% of IFN patients. Discontinuation due to neutropenia occurred in identical percentages as discontinuation due to anemia (2% in PEG, 0.2% in IFN). Serious psychiatric events were similar in the two treatment arms: suicide (none); suicide attempts (one patient in PEG 1.5 mg/kg arm, none in IFN arm); and suicidal ideation (suicide thoughts, 6 patients in PEG 1.5 mg/kg arm versus 7 in IFN arm). Discontinuation due to psychiatric events occurred among 6% of patients who received PEG 1.5 mg/kg and among 4% of the patients who received IFN. The overall modification rate of drug dosing (among patients in the upper ribavirin range) was 49% among PEG/RBV patients (42% receiving PegIntron 1.5 ug/kg/RBV) and 34% among IFN/RBV patients. When evaluating adverse events, there were fewer in the Pegasys/RBV arm than in the standard IFN/RBV arm in certain categories in the study presented by Michael Fried at DDW: myalgia 42% vs 50%, rigors 24% vs 35%, pyrexia 43% vs 56%, depression 21% vs 30%. For other adverse events the incidence reported was approximately equal in each group. We await further data from this study. At DDW, Robert Perrillo reported from a study of 412 treatment-naïve patients receiving Pegasys or standard IFN a-2b+RBV (1000/1200mg/day). 25% had cirrhosis (Knodell fibrosis score of 3 or 4). 84% were Caucasian, 73% genotype 1, 40% <10(6) IU/mL viral load, ALT was 110-112. At week 24 about 56% in both arms had negative PCR. Incidence of Most Common
Side Effects (Pegasys vs IFNa-2b+RBV) At week 12, patients receiving Pegasys (compared to patients receiving IFN/RBV) reported significantly less problems performing physical activities, better overall assessment of health & well-being, less reduced quality of life due to mental health and well-being, less distress over health and better positive well-being scores. Pegasys patients also reported better work productivity and less activity impairment: overall work impairment due to health, less activity impairment due to health, and less impairment while working due to health. Patients on Pegasys reported less weekly wages lost, less patients went from employed to unemployed (7% vs 18%). These data report improved safety and quality of life for Pegasys monotherapy over the first 12 weeks of therapy. Longer follow-up data is awaited. As well, post treatment data can be helpful. Tolerability may be more important to HCV/HIV coinfected patients for many reasons including: having to simultaneously take HIV HAART therapy; anemia in the setting of HIV may be more of a problem; adherence challenges may increase as coinfected patients will be taking more medications; coinfected patients may have lower threshold for tolerability and may be dealing with more issues. PegIntron tolerability data was reported at AASLD 2000. Ray Chung reported for NATAP: Abstract #590 found that health related quality of life measures were significantly better using the 0.5 ug/kg/wk PegIntron dose compared with conventional INTRON (standard interferon). Quality of life scores were comparable between PEG 1.0 and slightly worse with 1.5. The second abstract (#591) examined PEG-IFN-a-2a (Pegasys) given at a dose of 180 ug/wk for 48 weeks compared with the conventional Roferon regimen of 12 weeks of 6 MU tiw followed by 36 weeks of 3 MU tiw. Fatigue and quality of life indices were significantly better for Pegasys at weeks 2 and 12. These differences were seen at week 72 (24 weeks after stopping therapy), but were not statistically different. These data suggest that the side effect profile in the early going of therapy, usually the most difficult period of patients, will be better-tolerated. We await further long-term data regarding the efficacy of pegylated interferons with RBV in terms of tolerability. Again, there are no direct comparisons of the two PEGs in a study and the data we have on tolerability is preliminary. After using these drugs in the clinic, experience will reveal more about the tolerability and quality of life both on therapy and following treatment Achieving SVR Improves Fatigue, Quality of Life, General Well-Being & Functioning David Bernstein (North Shore Univ Hosp, Manhasset, NY) reported at DDW on a pooled analysis of 1400 patients from 3 large international studies that compared 3 Pegasys doses (90, ug, 135 ug, and 180 ug) with standard IFN a-2a (given either at one of two doses three times per week: 6 MIU for 12 weeks followed by 3 MIU for 36 weeks or 3 MIU for 48 weeks). Patients were monitored for 24 weeks after stopping therapy. In all patients with sustained viral response (SVR) and in those patients with SVR without cirrhosis, significant improvements on both measures of fatigue and all SF-36 scores were seen. SF-36 Health Survey evaluates health related quality of life (physical functioning, body pain, general health, vitality, social functioning, emotional & general mental health), positive well-being, distress over health, and overall physical and mental health. Patients with SVR with cirrhosis showed significant improvement in these evaluations, but not quite as much as the others achieving an SVR. Bernstein also reported that the patient’s responses to these evaluations of fatigue and other measures of well-being were predictive of patient discontinuation from treatment, suggesting that improved tolerability of treatment should help patients stay on therapy. Unfortunately, virologic nonresponders at week 72 reported worsened scores in fatigue and SF-36. Affect of HCV on the Brain, Fatigue, Hostility, and Anger Preliminary studies suggest that HCV can infect the brain just as in HIV. They also suggest that many people with HCV experience problems with fatigue, anger, hostility, and emotional distress. The affect of HCV on the brain may contribute to these other conditions. Coinfected patients may have a worse experience since viral and chronic diseases can contribute to this problem and both HIV and HCV can infect the brain. The presence of diabetes may also aggravate the condition. |
| < All newsletters |