 |
 |
 |
| |
Maternal - Fetal Transmission at Retrovirus
Written by Danielle Milano, MD, Boriken Family Health Services, New York
|
| |
| |
Judith Currier, M.D. of UCLA gave an excellent review lecture on Maternal-Fetal transmission (MFT) at Retrovirus (S #19). She outlined 3 major issues involved in prevention of MFT. Firstly, there is the obvious goal of decreased transmission to the fetus. Secondly, there are safety and efficacy issues for the mother. Short-term use of anti-retroviral's (ARV) do decrease MFT; however, development of resistance mutations is a concern. Lastly, there are safety concerns with long-term ARV exposure to the developing fetus.
There are now an increasing number of ARV options but optimal timing has yet to be defined when weighing the pros and cons to the mother and fetus of short-term use of ARV, long-term use of ARV, prevention of MFT, safety and efficacy.
The trial that started it all, ACTG076 showed that the use of AZT in the 3rd trimester drastically reduced MFT. Use of AZT + 3TC reduced the transmission rate further to <5%. The WITS trial, an observational database, showed that the number of anti-retroviral drugs in a regimen was inversely correlated with MFT. Mothers on no ARV had a 20% transmission rate. Use of 3-drug combination therapy reduced the MFT to < 5%.
Editorial note: some studies have shown rates as low as 1% MFT. The level of viral load of the mother is related to the risk of transmission. The lower the viral load the less liekly transmission will occur. A study showed that when women's viral load in this study were below 1500 there was no transmission, however, it is crucial to realize that transmission can occur and has been observed when the mother's viral load is below 500. So, HAART and undetectable viral load is not risk free, transmission can still occur.
The big question now is whether elective C-section (ECS) can reduce that transmission rate even further. Previously, ECS did reduce MFT if the mother was on AZT monotherapy, or on no ARV. With MFT rates dropping to <5%, it was no longer clear if ECS had any added benefit with if the mother was taking a 3 drug combination.
Data from PACTG 367 was presented in an oral abstract session (abstract 114). They looked at 2,087 pregnancies at 67 sites over 3 years, 1998-2000. Over the 3 years, the MFT rate dropped from 4.3% in 1998 to 1.6% in 2000 along with increases in third trimester multi-agent ART use. ECS rate increased from 12% to 29% over that same 3 year period. Overall, 88% of the women were in prenatal care starting in the 1st or 2nd trimester. 12% were not in care until the third trimester or until the time of delivery. 16% had an HIV-VL below the level of detection (BLD) at entry, and 50% were undetectable at delivery. Median CD4 was 392 cells/mm3 and median HIV-VL was 4,000 copies/mL. The overall transmission rate for the 3 year period was 3.6% (76 HIV+ infants). The transmission rate for 2000 was 1.6%.
The data was analyzed in a variety of ways. Using maternal ARV use as the only variable, the MFT rate was 20% in mothers not on ARV. For one agent alone (AZT or NVP monotherapy), the rate was 5.3%. Use of multi-agent ARV reduced the rate further to 1.8% (1.8% NRTI, 2.4% NNRTI, 1.6% PI).
Transmission varied by HIV VL: 6% (HIV VL > 10,000), 2.5% (HIV VL 10,000 to 1,000), 0.9% (HIV VL < 1,000). In women who did not have an HIV VL done at any time during pregnancy, transmission rate was 17%. These are the mothers who were not in prenatal care.
Analyzing MFT rates overall between ECS (2.6%) and vaginal delivery (4.2%), there was not a statistically significant difference. If you analyze the data using more than one variable, ECS does reduce MFT if the mother has not had an HIV-VL during pregnancy. If the HIV-VL is < 1,000 copies/mL, ECS does not lower the rate any further.
The poster from the Pediatric Spectrum of Disease Project (PSD) (#798-W) found similar results in most patient categories. As expected, combination-ARV resulted in a lower transmission rate when compared to monotherapy (4.9% vs. 9.4%). As expected, C-Section did not reduce MFT in women on combination-ARV. In contrast to PACTG367, they did not find a benefit to C-Section in women not on ARV-therapy. This data may be misleading since PSD did not look at elective C-Section versus emergent C-Section. It is known that events which dictate the need for emergency C-section are also associated with increased MFT. In short, if maternal HIV-VL is < 1,000 copies/mL or if the mom is on combination ARV, there is no benefit to having an ECS.
Moving back to Dr. Currier's talk, she then reviewed studies in the breast-feeding populations. The PETRA trial used AZT + 3TC at 36 weeks of gestation or at the time of labor. MFT was reduced by 38%. But the gains were all lost by 18 months secondary to breast feeding. HIVNET O12 which gave 1 dose of Nevirapine to the mom at labor and 1 dose to the infant within 72 hours showed a decreased MFT rate. This reduced rate persisted out to 12 months post-partum (24% vs. 16%). The point of these 2 studies is that short courses of ARV at or around the time of delivery can have an impact on MFT in the developing world. BMS094 in South Africa used mono-therapy nucleosides (D4T vs. ddI vs. AZT) or dual-therapy nucleosides (d4T/ddI) at 36 weeks of gestation and to the child immediately post-partum. MFT was lowest in the D4T/ddI arm (4.6%) and highest in D4T alone (11%). Overall MFT was 8%.
The concern is that resistance can develop with short-term single agent or dual agent use. 3TC and Neveripine both have low genetic barriers to resistance. 1 mutation confers resistance. In both HIVNET 012 and PACTG 316, Nevirapine mutations were detected 6 weeks post-partum but were no longer measurable 1 year later. The concern is that resistant virus may be archived and may be associated with an increased risk of transmission to the fetus. In ACTG 076, AZT monotherapy resulted in low-level AZT resistance in 0.3 to 14% of the mothers. AZT resistance was associated with an increase in MFT rate in the WITS cohort.
Thus, there is a concern that we may be compromising the mom's future ARV options with short course therapy.
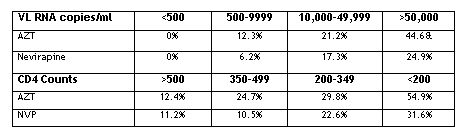
Editorial note: In HIVNET 012 women received either nevirapine or AZT. This study was a phase II randomized open-label study in Uganda demonstrating the effectiveness of the 2-dose nevirapine regimen given to HIV-infected mothers at labor onset and to their neonates compared to intrapartum and 1 week postpartum AZT for reducing perinatal transmisiion. At retrovirus (abstract 120) researchers reported a sub-analysis of the 18-month transmission rate in HIVNET 012 by maternal viral load and CD4 count.
18-Month Transmission Rates (%) By Viral Load and CD4
|
|
 |
| |
Safety
Dr. Currier briefly covered changes in pharmacokinetics (PK) in pregnancy. There are increases in total body water and fat which affect the volume of distribution of drugs. Renal and cardiac blood flow increase as does GI transit time. AZT & 3TC PK is not changed in pregnancy. Nevirapine half-life decreases but the change is not clinically significant. Ritonavir 600 BID achieves adequate blood levels but is poorly tolerated by the mother and placental transfer is low and result in low levels in the fetus (Abs 794-W). Nelfinavir is well tolerated by the mother and likewise has low placental transfer (ABS 795-W).
Efavirenz (Sustiva) results in severe birth defects in monkeys and should not be used. Hydroxyvrea is also unsafe for use in pregnancy. Taking this a step further, it is my own recommendation that neither Hydroxyurea nor Sustiva should be used in HIV+ women of child bearing age who many be considering pregnancy or are not using contraception.
Overall, the ARV Pregnancy Registry (1-800-258-4263 or www.apregistry.org) shows a rate of 2.3% for adverse events or birth defects. This is not different than the rate for the general HIV- population. The data on pre-term labor is conflicting. PACTG 367 did not show an increase of pre-term labor in over 1,000 pregnancies, where as the European Collaborative and the Swiss-Mother-Child Cohort both did. HIV itself can cause pre-term labor so the jury is still out.
Lastly, Dr. Currier reviewed adverse events associated with ARV in general. Lactic acidosis and hepatic steatosis are more common in women on ARV. Of the 100 cases of Lactic Acidosis reported to the FDA, 83% were in women. 3 deaths have occurred in pregnant women on D4T/ddI: the women were on those 2 drugs for their entire pregnancies.
Editorial note: (abstract 113) a prospective, observational study was conducted by Alimenti to investigate the potential mitochondrial toxicity of antiretroviral exposure during the perinatal period in HIV-uninfected infants by determining lactate levels. Previous studies show normal lactate beyond the first week of life in infants not exposed to HAART. Plasma lactate was measured repeatedly during the first 6 months of life in a consecutive cohort of 25 infants exposed to HAART in utero and zidovudine during the neonatal period. Lactate was above normal limit at 2.1 mmol/L (>1 measurement) in 23/25 (92%) infants and reached levels >5 mmol/L in 9/25 (36%). The hyperlactatemia was persistent, resolving by the age of 6 months
in most infants. 1 infant had symptoms consistent with those of adult lactic acidemia. HAART consisted of 2 NRTIs (ZDV/3TC most often) and nevirapine (20/25) or a PI (5/25). The mean duration of HAART exposure in utero was 17 weeks (range 3.5-38). Half of the infants were co-exposed to heroin, cocaine, or methadone. Maternal lactate level was normal in 17 mothers tested at the end of pregnancy. No association was found between the infant peak lactate and the duration of HAART during pregnancy (mean 4.2 vs 4.1 for <20 vs >20 weeks, p=0.9), exposure to stavudine (p=0.6), nevirapine vs PI-containing regimens (p=0.2) or co-exposure to substance use (p=0.3).
Authors concluded- this is one of the first prospective observational studies describing lactic acidemia in HIV uninfected infants exposed to HAART during the perinatal period. The hyperlactatemia was noted in 92%
of the infants, and was above 5 mmol/L in one third. We hypothesize that the persistent hyperlactatemia is a consequence of mitochondrial toxicity from the transplacental and neonatal exposure to antiretroviral
agents, as well as of impaired hepatic lactate clearance. Further study is required to determine the factors impacting on the hyperlactatemia and its clinical significance. Clinical follow-up and monitoring of lactate
levels is recommended during the first 6 months of life in all infants exposed to perinatal HAART.
Impaired glucose tolerance (IGT) and diabetes mellitus (AODM) are associated with pregnancy and also with Protease Inhibitor therapy. Pregnant women in one small study did have an increased rate of gestational diabetes (3.5%) when compared to HIV- women. Glucose Tolerance Tests are routinely monitored during pregnancy, therefore any increased prevalence will be detected.
Putting it all together, the recommendations are:
- Use a fully suppressive 3-drug ARV regimen.
- C-Section is not necessary if the mother's HIV-VL is <1000 copies/ml
- Have a low threshold to check for therapeutic drug levels.
- Have a low threshold to perform resistance testing.
- Delay ARV until at least 12 to 14 weeks of gestation to decrease teratogenicity but there is no clear best time to initiate therapy.
Lastly, there is no clear advice for the woman already on ARV who comes in pregnant. Clearly, those on Sustiva, hydroxyurea, or the ddI/d4T combo should be switched or the therapy discontinued.
|
|
| |
|
 |
 |
|
|