 |
 |
 |
| |
Atazanavir: The Story Unfolds with Effectiveness Comparable To Efavirenz
Reported by Steve L Becker, MD
|
| |
| |
As the atazanavir clinical development program concludes its registrational trials, a series of studies, both of clinical effectiveness as well as drug interaction and pharmacokinetics are being reported. The most recent reported at the 42nd ICAAC by Kate Squires (abstract H1076 ) recounts the final 48 week data of atazanavir (ATV) versus efavirenz (EFV) both paired with zidovudine and lamivudine (Combivir). This trial occurs against the backdrop of the previously reported phase II ATV trial against nelfinavir (ref). In that trial, with a stavudine and lamivudine NRTI backbone, results suggested only modest activity of both regimens (on treatment analysis < 400 copies of only 74% for ATV, 69% NFV). The current trial was conceived to compare ATV against the arguable standard of care, EFV/Combivir in treatment naive patients. An ongoing trial of ATV vs. lopinavir/ritonavir (Kaletra) in treatment experienced patients will be reported next year.
The current trial involved 810 treatment naive patients, of whom 35 % were female, 67% non-Caucasian, and was conducted at multiple international sites with 60% of patients from non-U.S. or Western European countries. Patients had to have CD4 > 100 cells and HIV RNA > 2000 copies/ml. Patients were randomized in a 1:1 fashion to receive ATV 400mg + Combivir + EFV placebo or EFV 600mg + Combivir + ATV placebo. The study's primary endpoint was the % of patients with HIV RNA < 400 copies, while secondary endpoints included the % achieving < 50 copies, CD4 count rise, fasting cholesterol and triglyceride levels, fasting glucose and insulin levels as well as safety and tolerability. The statistical design of the study was for non-inferiority, meaning that ATV was assumed to be non-inferior to EFV. The design was appropriate for this type of study.
Patients in this study were well matched between the two treatment arms and relatively advanced in their HIV disease. Mean HIV RNA was 4.89 log10 copies/ml (about 85,000 copies/ml) and mean CD4 count was 280 cells/mm3. Fully 42% of patients had HIV RNA levels > 100,000 copies/ml. This is important as it allows the potency of the regimen to be compared against itself in advanced and less advanced patients, as well as against the competitor arm. 15% of ATV and 13% of EFV treated patients were HCV or HBV co-infected. 82% of patients completed 48 weeks of study. 7% of patients discontinued because of adverse events, while only 2% discontinued because of investigator defined virologic failure. Patients who were intolerant to either component of Combivir were not allowed to switch to another NRTI combination, and were therefore dropped from the study. This may have lead to a greater dropout rate and influenced the intent to treat analysis.
Using the newer FDA definition of treatment success, called 'responders', patients had to have remained below the level of detection (either 400 or 50 copies/ml) for the duration of the 48 week study. Patients with HIV RNA levels above the virologic endpoint on two consecutive occasions were defined as failures. In practice this means that a patient with HIV RNA values of 51 and 52 copies/ml on successive viral load determinations is considered a treatment failure even if all other viral load measures were < 50 copies. This 'responders' method of determining treatment effectiveness differs from the historical approach where HIV RNA levels were reported at a specific study time point, usually 24 or 48 weeks. The advantage of the newer method is that patients with viremia at time points other than the specified 24 or 48 week time point would be counted as treatment failures even if their viral load reached a value below quantitative limits at week 48. This method is more conservative and will yield lower rates of success than the previous methods of reporting. The disadvantage lies in the variability of viral loads and the assays used to measure HIV RNA. In the case of defining success as < 50 copies, the biological and assay variability may allow a patient to rise above 50 copies, but then revert to levels < 50 for the remainder of the study. It would be hard to call a patient with HIV RNA measures of 51 and 52 copies a treatment failure, yet that is exactly what this newer definition of success would do. The tables below show the % of patients < 400 and < 50 copies by intent to treat (ITT) where missing values equal failure (M=F) and by an on treatment analysis.
ITT, M=F
< 400 copies
< 50 copies
ATV + Combivir + EFV placebo
70%
32%
EFV + Combivir + ATV placebo
64%
37%
|
|
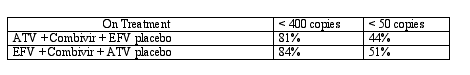 |
| |
These results are obviously very similar, and ATV is technically non-inferior to EFV, though formally not equivalent (study design would have had to be for equivalence, requiring a larger sample size). None of the viral load differences between the ATV and EFV arms reached statistical significance. Response rates among women were comparable to those for men, perhaps a little better.. When looking at the response in patients with greater than or less than 100,000 copies, no difference in the ATV or EFV arms was present whether evaluating by <400 or <50 copies/ml. The rise in CD4 counts was 176 for ATV and 160 for EFV treated patients.
Grade 2-4 adverse event rates were low in both study arms. Jaundice and scleral icterus, known side effects of ATV, were reported in 5% and 1% of patients, respectively. Markers of hepatic toxicity (AST and ALT) were not different between the groups, nor were white blood cell counts or hemoglobin. Total bilirubin elevations > 2.5 were reported in 33% of ATV treated patients. This hyperbilirubinemia has been reported in previous ATV studies, and reflects a genetic defect in bilirubin metabolism (the urdine diphosphate-glucuronosyl transferase, UGT1A1, enzyme system). The elevated bilirubin levels, often present before drug administration, are without health consequence and resolve promptly upon discontinuation of the drug. The hyperbilirubinemia seen with ATV is similar in origin, with allelic variants in UGT1A1, to that seen in patients treated with indinavir.
Diarrhea 1-2%. Nausea 13-14%. Rash: 6% ATV, 10% EFV. Headache 5%. Dizziness; 2% ATV, 6% EFV.
The lipid profile of ATV has been noted to be unlike other protease inhibitors where total cholesterol, low density cholesterol (LDL) and triglycerides are increased by 20-40% above pretreatment levels. This study again demonstrated favorable lipid findings. The fasting lipid profiles of ATV and EFV are shown below.
|
|
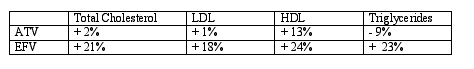 |
| |
(editorial note: glucose was 90 at baseline for ATV group and 93 at week 48, and for EFV group it increased from 90 to 94. Fasting insulin increased significantly for both groups but the clinincal relevance of this increase is not known: 11.3 to 12.3 for ATV group & 9.9 to 11.5 in EFV group).
In a preliminary analysis of resistance mutations noted in those with virologic failure most patients had evidence of the M184V. Genotypes of ATV treated patients showed the characteristic (signature) I50L substitution in 3 of 25 patients. This substitution has been recently described as unique to ATV, and resulted in hypersusceptibility to all other PIs (Colonno, Seville, 2002). Among those failing the EFV based regimen, a high percentage, 13 of 20 (65%), showed the characteristic K103N substitution. Further characterization of failure patterns from ATV treated patients will be forthcoming, but observations from this study confirm the I50L findings from other trials conducted in treatment experienced patients. 30% of patients receiving EFV had AZT mutations (41, 70, 210, 215) and 16% receiving ATV had these mutations.
The results of this study are noteworthy. The fact that ATV appears similar in effectiveness to EFV is impressive. EFV with dual NRTIs, whether Combivir, or lamivudine (3TC) and tenofovir as reported in the recent Gilead Sciences trial, is rightfully the current standard of care. That ATV would be found to have similar effectiveness to EFV will surprise many. The success rate in this trial is however lower than many previous EFV studies. What might account for the apparent difference in performance? Clearly the definition of treatment effectiveness is one factor. Prior studies used a less stringent definition of success. Another factor may be the viral load assay used in this study. Because patients were enrolled from Asia, Africa and South America, an assay capable of detecting clades (strains) other than clade B, the predominant strain in the U.S. and Western Europe, was necessary. The assay used in this study, Roche Amplicor version 1.5, detects virus with greater sensitivity than the conventional PCR assay. Some patients evaluated by version 1.5 showed detectable virus, where if used, version 1.0 would have read undetectable. In a substudy of the trial undertaken in hundreds of patients, use of the
version 1.5 assay resulted in detection of HIV-1 RNA in 12% of patients whose
viremia had been below the
level of detection of the version 1.0 assay. Reported success rates may have been lowered because of this. Finally, it appears that performance in some geographic regions was worse than others. The lowest rates of success were noted at the European sites. This may have reduced the overall performance of both study arms.
Finally how can the clinician and community account for the relatively modest effect of ATV when tested against NFV and the success against EFV? Clinical studies do not always yield the same results. The scientific method requires confirmation of research findings, and contradictory results often occur. Study populations may differ, and as a rule cross study comparisons should be avoided. The true effectiveness and utility of ATV may not be known until it is used more widely, both in subsequent clinical trials and in the community.
Despite the caveats noted above, this study surely places atazanavir among the agents to be considered early in HAART therapy. While its effectiveness in treatment naive patients when tested against nelfinavir was less impressive, the current study should prompt reconsideration of ATV's potency. Results of the treatment experienced trial against Kaletra are eagerly awaited to more fully define the place in HIV treatment for this agent. Meanwhile, the low pill burden (2 per day), single daily dosing, favorable lipid and glucose profiles, and, to date, novel resistance pattern suggest that ATV will find a place in treatment of HIV infected patients requiring a protease inhibitor. Further studies of combining ATV with other PIs, the use of ATV with the NNRTIs EFV and NVP which induce ATV metabolism and thus lower ATV exposures, as well as important drug interaction studies are underway. These datasets will likely be completed and reported at upcoming meetings prior to the anticipated approval of ATV in mid-2003.
|
|
| |
|
 |
 |
|
|