 |
 |
 |
| |
Preliminary Results from Comparison of PegIntron 1.0 ug/kg vs PegIntron 1.5 ug/kg
Reported by Jules Levin
|
| |
| |
Steve Flamm reported preliminary study results from a comparison of two doses of PeginTron plus ribavirin: PegIntron 1.5 ug/kg/wk plus ribavirin compared to PegIntron 1.5 ug/kg/wk plus ribavirin; 1.5 ug/kg/wk is the FDA approved dose for combination therapy. PegIntron monotherapy is approved at the 1.0 ug/kg/wk dosage. Toxicity and tolerability are thought to be dose related. But the sustained viral response (SVR) of PegIntron 1.0 ug/kg/wk plus ribavirin is unknown.
Patients had chronic HCV, were treatment-naive, normal or abnormal ALT, had compensated liver disease, without concomitant liver disease, without renal failure, without other uncontrolled medical or psychiatric disease, and without contraindications to PegIFN or ribavirin.
The study is randomized, controlled, multi-center, and patients were randomized to one of 2 treatment arms:
--PegIntron 1.0 ug/kg/wk plus ribavirin (800mg-1400mg/day)
--PegIntron (1.5 ug/kg/wk) plus ribavirin (800mg-1400mg/d)
Therapies were administered for 24 weeks for genotype 2/3 and for 48 weeks for genotype 1/4. Medications were discontinued in genotype 1/4 patients after week 24 if HCV RNA was detectable. Medications were discontinued at week 12 if there was not a 2-log reduction in viral load at the discretion of the doctor. Histologic informationwas obtained prior to starting study therapy. Tolerability was followed (medication dose reduction, adverse events, and drop-out rates).
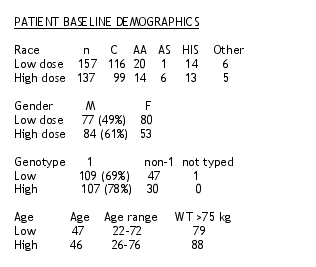
294 patients were enrolled in the study.
|
|
 |
| |
RESULTS
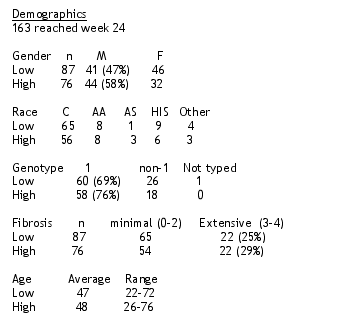
11 patients were randomized and did not receive medication. 121 patients have not yet reached the 24-week timepoit. 163 patients have either reached the 24-week time point or were discontinued prior to 24 weeks. These patients are included in the analysis.
|
|
 |
 |
 |
| |
CONCLUSIONS by Authors
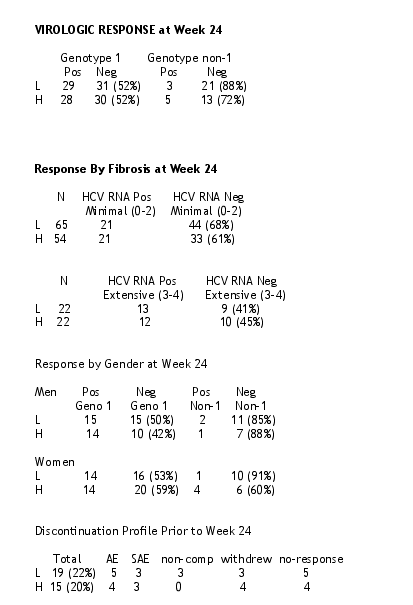
--PegIntron 1.0 ug/kg/wk plus ribavirin is equally effective as 1.5 ug/kg/wk plus ribavirin in regards to HCV RNA negativity at week 24.
--Equivalent treatment responses were noted in genotype 1 vs non-I genotype, men vs women and minimal vs extensive fibrosis.
--Medication discontinuations were equal between the two groups.
--Adverse events and severe adverse events requiring discontinuation of the drugs were equal between the two groups.
--Adverse events requiring dose reduction were less in the low dose group.
Comments by Jules Levin
I think these results are too preliminary to draw strong conclusions. The data is not complete, week 48 and 72 data has not been compiled. When Roche compared Pegasys 135 mcg/wk to the approved full dose of 180 mcg/wk the antiviral response was comparable but there was histologic benefit to the full 180 mcg dose. I hope that Schering will conduct a large enough study to address this question.
|
|
|
 |
 |
|
|