 |
 |
 |
| |
FUZEON: IMPACT OF ENFUVIRTIDE (Fuzeon) ON HEALTH-RELATED QUALITY OF LIFE AT 48 WEEKS
|
| |
| |
Poster at 9th European AIDS Conference, Warsaw, Poland, October 2003
Clumeck N1, Cohen CJ2, Thompson M3, Molina J-M4, Patel K5, Wintfeld N5 and Green J5. 1 Centre Hospitalier Universitaire Saint-Pierre, Brussels, Belgium; 2 Community Research Initiative of New England, Boston, MA, USA; 3 AIDS Research Consortium of Atlanta, Atlanta, GA, USA; 4 Saint-Louis Hospital, Paris, France; 5 Roche, Nutley, NJ, USA
INTRODUCTION
HIV infection and the adverse effects of antiretroviral therapy (ARV) can affect health-related quality of life (HRQoL).
Enfuvirtide (ENF), the first HIV--fusion inhibitor, has shown potent and sustained antiviral activity when administered in combination with optimized background (OB) therapy to treatment-experienced, HIV-1- infected individuals and has a favourable safety profile.
In Phase III trials, ENF + OB was associated with significantly greater treatment benefit than OB alone over 48 weeks. Patients randomized to ENF + OB achieved a mean reduction from baseline in plasma HIV-1 RNA of 1.48 log10 copies/mL at 48 weeks, compared with 0.63 log10 copies/mL for patients randomized to OB alone [intent-to-treat (ITT) analysis, last observation carried forward (LOCF)]. This was accompanied by a mean increase in CD4+ count of 91 cells/mm3 in patients randomized to ENF + OB, compared with 45 cells/mm3 in the OB alone arm.
ENF is administered by subcutaneous (SC) injection (90 mg bid), whereas other currently approved HIV medications are taken orally.
Local injection site reactions (ISRs) are the most frequent adverse events (AEs) associated with the use of enfuvirtide; however, over 48 weeks of therapy few patients (< 5%) discontinue ENF for this reason.
Other than ISRs, the AEs most frequently reported in subjects receiving ENF + OB were also commonly observed in subjects who received OB alone. Rates of constitutional AEs, e.g. diarrhoea, nausea, fatigue, headache, insomnia and vomiting, were lower on ENF.
The Medical Outcomes Study (MOS)--questionnaire is a well-established and comprehensive measure of HRQoL. Twenty-four-week data from patients enrolled in the TORO trials were reported previously and showed that self-injected ENF added to an OB regimen does not adversely affect and may improve HRQoL when self-administered by treatment-experienced, HIV-1-infected individuals.
STUDY OBJECTIVE is to investigate the impact of ENF treatment on HRQoL during 48 weeks of treatment.
Author Summaries
For most scales, an improvement in HRQoL was seen both in patients receiving ENF + OB and in those receiving OB alone at all time-points. Although this study was not designed to compare changes in MOS-HIV scores over time, it is interesting to note that most measures appeared to improve on-study in both treatment groups. This suggests that optimizing background therapy prior to enrolment in a clinical trial, in itself, tends to improve HRQoL. Therefore, it would be expected that ENF, in addition to an individualized background regimen, should provide an even greater benefit to patients when measuring HRQoL outcomes.
Indeed, as hypothesized, nearly all scale scores and both summary scores were greater in the ENF + OB group than the OB alone group, both at week 24 and at week 48. Four out of the ten scale scores as well as the mental health summary score showed a statistically significant difference in change from baseline compared with OB alone at these time-points. This suggests that most patients continue to enjoy HRQoL benefits associated with ENF treatment over the long term.
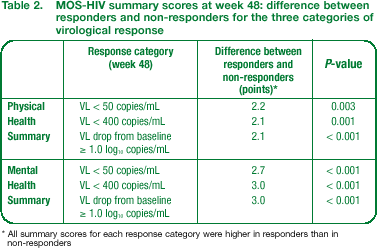
This study was not designed to ascertain the causes of any HRQoL benefits associated with the initiation of ENF therapy. However, it could be speculated that the HRQoL benefits seen with ENF might be related to virological and immunological responses, which showed greater improvement over baseline in the ENF + OB group compared with OB alone in both TORO trials. In other trials, improved HRQoL scores have been shown to correlate with increased CD4+ cell count and decreased viral load, as well as with patient continuation of therapy. In the TORO studies reported here, HRQoL improvements were greater in virological responders compared with non-responders.
I reported 3 poster studies to you on Fuzeon, which were reported at the European AIDS Conference. I reported to you the poster at EACS reporting the results of the patient survey which assesses the effect of self-injection on patient functioning and daily activity, and the ease or difficulty of self-injection. And I reported the results from a study evaluating the effects of baseline demographics (gender, ethnicity, baseline viral load, CD4 count, etc) on the efficacy of Fuzeon. Taken together, these posters show Fuzeon is effective in reducing viral load and increasing CD4 counts for patients with a strong degree of resistance to the currently available classes of HIV drugs (NRTIs, NNRTIs, PI). Most patients are able to effectively self-inject. The effects of successful Fuzeon therapy result in improved health. New treatments including new entry inhibitors are in development and are expected to be available within several years, so Fuzeon can be helpful in transitioning to that time for individuals with limited treatment options now.
Study design and participants
HRQoL was assessed in two Phase III, randomized, controlled, open-label trials of ENF + OB vs. OB alone (TORO 1 and 2).
Trial participants were HIV--infected adults with at least 6 months (TORO 1) or 3 months (TORO 2) prior experience of and/or documented resistance to each of the three non-fusion inhibitor classes of approved antiretrovirals and plasma HIV-1 RNA >5,000 copies/mL at baseline.
995 individuals enrolled in the TORO trials received ENF (90 mg bid SC)) plus an optimized background (OB) regimen or OB alone and had at least one follow-up visit (ITT population).
Patients initially randomized to OB alone who experienced protocol-defined virological failure after week 8 were allowed to switch to receive ENF plus a revised OB regimen (switch patients). HRQoL measurement
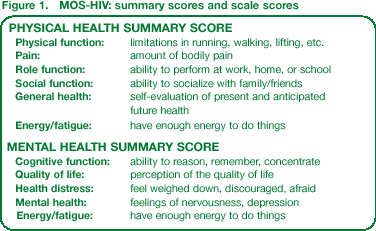
Participants completed a Medical Outcomes Study HIV Health Survey (MOS-HIV) questionnaire at baseline, 4, 8, 16, 24 and 48 weeks (Figure 1).
This instrument has shown good internal consistency, test–retest reliability and validity, and is able to detect clinically important differences in HRQoL, including functional status and well-being.
As the TORO trials involved patients from several countries,, authenticated local language versions of the MOS-HIV were used where appropriate.
To ensure that patients responded independently of the clinical evaluation at each time-point, patients were asked to complete the MOS-HIV before any clinical procedures or consultations at each visit.
48-week data from the two trials were pooled for the secondary HRQoL analyses presented here, since the study designs, patient selection criteria and baseline characteristics for the trials were very similar.
Data analysis
Analysis of covariance (ANCOVA) was used to evaluate changes from baseline in the ten MOS-HIV scale scores and two summary scores (physical health and mental health).
Treatment comparisons (intent--treat) were made, adjusting for randomization, MOS-HIV baseline score, protocol-defined stratum [four categories based on viral load at screening (< 40,000 or >40,000) and whether or not newly approved/investigational ARV agents were part of the patient’s OB treatment regimen] and treatment–stratum interaction.
Data analysis was performed using the ITT population. For patients who either withdrew from the study, experienced virological failure (as defined in the clinical trial protocol) or who were switch patients, MOS-HIV scores were censored at the time of withdrawal or failure and missing values imputed using the LOCF method.
Least squares means were used to test for treatment group differences and difference between virological responders and non-responders. Categories of virological response were defined as < 50 copies/mL, <400 copies/mL and <1.0 log10 copies/mL drop in plasma HIV-1 RNA from baseline.
|
|
| |
| |
 |
|
| |
| |
RESULTS
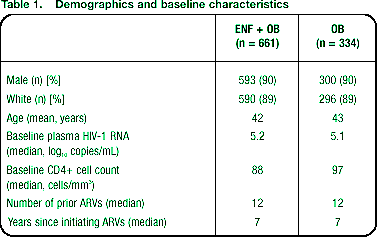
Baseline demographics are shown in Table 1..
At baseline there were no significant between-group differences in any HRQoL measure.
In the 48--ITT (LOCF) analysis, MOS-HIV observations were available for 98.5% of study participants.
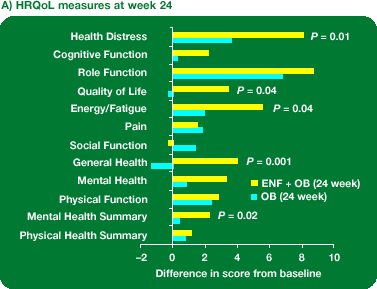
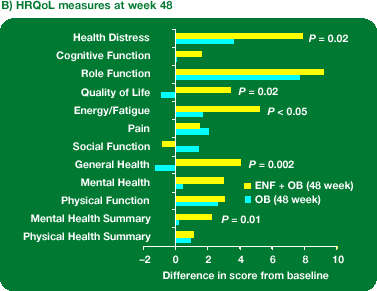
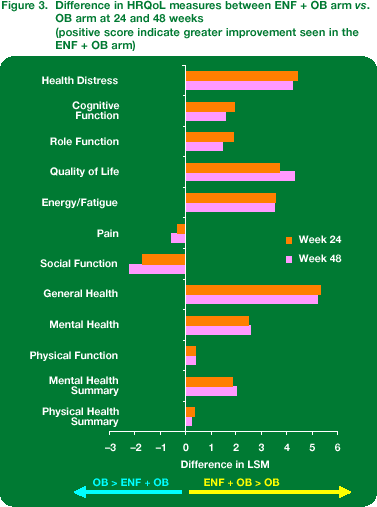
At week 48, for eight out of ten MOS--subscale scores and both summary scores the difference in change from baseline favoured ENF + OB (Figure 2 and Figure 3).
Scores for general health, energy//health distress, quality of life scale and mental health summary were statistically significantly higher with ENF + OB than with OB alone (P < 0.05).
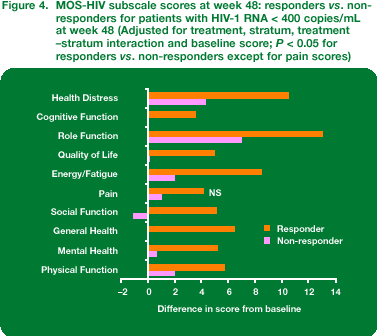
Based on all three protocol-defined categories of virological response, responders in both the ENF + OB and OB arms combined had significantly greater improvement in HRQoL summary scores, as well as most HRQoL subscales, than non-responders (P < 0.01) (Table 2; Figure 4 – data shown for < 400 copies/mL only).
|
|
| |
| |
 |
|
| |
| |
The general health scale score (see Figures below) showed the most immediate and durable difference between ENF + OB and OB alone; improvements in this subscale were statistically significantly higher in the ENF arm than in the OB arm at all post-baseline time-points (4, 8, 16, 24 and 48 weeks)
Social function appeared to be the parameter least favourably affected by the addition of ENF. In the ENF + OB group, this was one of only two parameters to show a negative change in least squares mean from baseline. By contrast, there were no negative changes in this parameter in the OB group. Nevertheless, none of the between-group differences in social function reached statistical significance at any time-point.
The only other measure where a greater improvement was seen in the OB group compared with the ENF + OB group was the pain scale score, although the difference was not statistically significant at either 24 or 48 weeks.
Figure 2. HRQoL measures
|
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
|
|
| |
|
 |
 |
|