 |
 |
 |
| |
Hypophosphataemia in Patients Taking Tenofovir
|
| |
| |
Reported by Jules Levin
Researchers from the UK reported at the9th EACS HIV/AIDS Conference in Europe (Warsaw, Poland, Oct 25, 2003) on a study examining if there is an association between hypophasphatemia and tenofivir. This study was probably prompted by recent reports of patients taking tenofovir experiencing hypophosphataemia either isolated or in association with Fanconi syndrome and acute renal failure. The study was reported by Day and colleagues (Sussex University Hospitals NHS Trust, Brighton, UK 2 Centre for Health Care Research, University of Brighton, Brighton, UK).
Summary. Data were obtained on 259 patients. The frequency of HP (hypophosphatemia) (SP<0.8mmol/L) in groups A, B, C and D was 30.7%, 22.7%, 9.8%, and 10.5% respectively. Group A - taking TDF-containing HAART regimen. Group B - taking TDF-sparing HAART regimen. Group C - naïve to antiretroviral therapy (ART). Group D - treatment experienced but not taking HAART. By multivariate analysis, the only factors found to be independently associated with HP were: cumulative time on HAART (p<0.007) and currently taking Kaletra (p<0.026). There was no significant association found between HP and: Age, TDF, HIV disease stage, as estimated by nadir CD4 cell count, Concurrent use of nephrotoxic agents, Abnormal markers of renal function.
Authors concluded: despite observing a higher frequency of HP in TDF recipients than was seen in clinical trials, no significant association with TDF was identified. Cumulative time on HAART was strongly associated with HP, suggesting that the higher frequencies observed may be a reflection of 'salvage' therapy. These results accord with the differing HP frequencies previously observed between HAART naïve and experienced patients. It has been suggested that concurrent Kaletra may increase the risk of renal toxicity of TDF, which is biologically plausible given that it produces a 30% increase in CMax and AUC of TDF 13. However, we found Kaletra to be independently associated with HP, increasing the risk by five fold. The range of HP observed in all groups appears mild to moderate, which may imply a low risk of developing clinically relevant bone pathology. Although Cheng et al found HP to be a transient biochemical abnormality, resolving without treatment interruption, our reproducibility figures suggest it is likely to persist in the majority of cases, though it may not be related to TDF per se. In patients with HP we recommend repeat SP measurements and evaluation of clinical status before withdrawing any medication.
In the absence of any apparent clinical consequences treatment continuation may be appropriate. If HP is persistent, severe or symptomatic in a patient with limited HAART options, phosphate replacement may be an option 2, and investigations to identify alternative causes of HP and associated renal dysfunction should be considered.
Background
Toxicity is a major factor limiting response to Highly Active Antiretroviral Therapy (HAART).
Adefovir, a compound of similar structure to tenofovir disoproxil fumarate (TDF), was discontinued as HIV therapy due to proximal renal tubule dysfunction1.
There have recently been reports of patients taking TDF experiencing hypophosphataemia (HP) [either isolated 2 or in association with Fanconi syndrome 3,4,5,6] and acute renal failure 4,5,6,7,8.
Preclinical studies observed renal toxicity and bone pathology (reduced bone mineral density potentially mediated by HP and osteomalacia) in animals taking high levels of TDF. 9 . Clinical studies identified a high frequency of mild HP (16%) amongst HAART experienced TDF recipients, and reduced bone mineral density in naïve TDF recipients. No clinical studies have observed definitive renal toxicity or grade 3-4 HP. 10,11,12
Due to concern that HP may reflect TDF-mediated renal toxicity, or lead to clinically relevant bone pathology, this department has routinely measured serum phosphate (SP) in TDF recipients, as recommended in the product Summary of Product Characteristics. 13
Objectives
To identify and compare the frequency and severity of HP in clinical practice amongst the following patient treatment groups:
- TDF-comprising HAART
- TDF-sparing HAART
- Not receiving HAART (both therapy naïve and experienced)
To assess the reproducibility of HP in patients taking TDF.
To evaluate the role of other disease factors in the aetiology of HP.
To identify any association between HP and:
- Abnormal markers of renal function
- Concurrent use of Kaletra
- Concurrent or prior use of nephrotoxic agents.
Method
HIV positive ambulatory care patients underwent serum phosphate testing:
- Group A - taking TDF-containing HAART regimen
- Group B - taking TDF-sparing HAART regimen
- Group C - naïve to antiretroviral therapy (ART)
- Group D - treatment experienced but not taking HAART
Patients in groups B, C and D had a single SP measurement, whilst those on TDF were monitored regularly as part of their routine clinical care.
The following data were recorded in all groups: nadir CD4 cell count; time since HIV diagnosed; CD4 cell count, HIV RNA viral load (VL), serum urea and creatinine taken at the time of SP; duration taking TDF; cumulative duration on ART; concomitant use of Kaletra and history and/or concurrent use of nephrotoxic agents.
Results
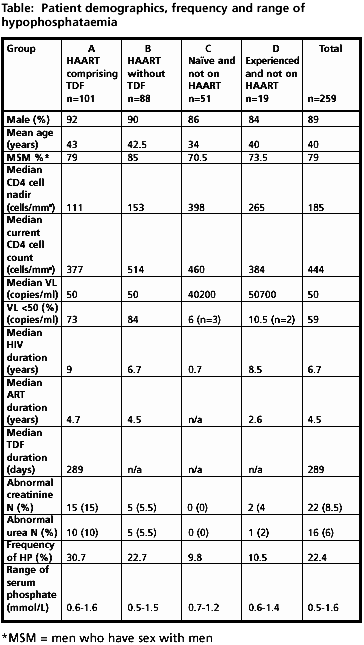
Demographics: Data were obtained on 259 patients, with 101, 88, 51 and 19 patients in groups A, B, C and D respectively. Gender, age, sexuality, surrogate markers, duration taking TDF and/or HAART and markers of renal function in each group are shown in table. Table is below and contained in article posted today on NATAP website, cannot include in email.
Frequency of HP:
The frequency of HP (SP<0.8mmol/L) in groups A, B, C and D was 30.7%, 22.7%, 9.8%, and 10.5% respectively.
Range of serum phosphate:
Group A (HAART w/TDF) n=101: 0.6-1.6 mmol/L Group B (HAART w/o TDF) n=88: 0.5-1.5 mmol/L Group C (naïve & not on HAART) n=51: 0.7-1.2 mmol/L Group D (experienced & not on HAART) n=19: 0.6-1.4 mmol/L
Reproducibility of HP: 44/101 (44%) of patients taking TDF received multiple serum phosphate measurements, with reproducibility of results observed in 76%.
Markers of associated renal disease:
Patients with abnormal urea (>7mmol/L) and creatinine (>100mmol/L) are shown in the adjacent table.
Factors associated with HP
Univariate analyses showed a significant association with HP and
- Patients taking HAART (p<0.004, X2 8.48)
- Increased duration of HAART (p 0.001, t= -3.25)
- Concurrent use of Kaletra (p<0.005, X2 7.72)
- Increased time since HIV diagnosis (p<0.033, t= -1.83)
- History of nephrotoxic agents (p<0.023, X2 , 5.154)
However, there was no significant association found between HP and
- Age
- TDF
- HIV disease stage, as estimated by nadir CD4 cell count
- Concurrent use of nephrotoxic agents
- Abnormal markers of renal function
By multivariate analysis, controlling for age, time since HIV diagnosis, HIV disease stage, cumulative HAART duration, use of TDF, Kaletra and history of nephrotoxic agents, the only factors found to be independently associated with HP were
- Cumulative time on HAART (p<0.007)
- Currently taking Kaletra (p<0.026)
Discussion by authors
In this study, despite observing a higher frequency of HP in TDF recipients than was seen in clinical trials 10, no significant association with TDF was identified. Cumulative time on HAART was strongly associated with HP, suggesting that the higher frequencies observed may be a reflection of 'salvage' therapy. These results accord with the differing HP frequencies previously observed between HAART naïve and experienced patients 10,12.
It has been suggested that concurrent Kaletra may increase the risk of renal toxicity of TDF 6, which is biologically plausible given that it produces a 30% increase in CMax and AUC of TDF 13. However, we found Kaletra to be independently associated with HP, increasing the risk by five fold.
The range of HP observed in all groups appears mild to moderate, which may imply a low risk of developing clinically relevant bone pathology.
There was no significant association between HP and abnormal urea or creatinine, suggesting that there may be separate mechanisms causing these biochemical abnormalities, (e.g. intestinal phosphate loss rather than renal wasting) 9.
Alternatively, creatinine may be an insensitive marker of abnormal renal function in HIV patients with reduced muscle mass.
Previous authors have found that a prior history of renal toxicity with adefovir or cidofovir may predispose TDF recipients to recurrent HP 2. However, this study found no association between history/current use of nephrotoxic agents and HP using multivariate analysis.
Although Cheng et al 10 found HP to be a transient biochemical abnormality, resolving without treatment interruption, our reproducibility figures suggest it is likely to persist in the majority of cases, though it may not be related to TDF per se.
Conclusions by authors
The aetiology (cause) of HP needs further evaluation but appears to be multifactorial. A similar scenario was seen with lipodystrophy 14, a condition initially attributed to HAART alone, but subsequently determined to be associated also with features of HIV infection and host factors.
Many patients using Kaletra and TDF who are found to have HP are highly treatment experienced and may have few alternative options. Introducing (and potentially over-interpreting) new monitoring tools without thorough evaluation may result in the unnecessary or premature discontinuation of medication.
In patients with HP we recommend repeat SP measurements and evaluation of clinical status before withdrawing any medication. In the absence of any apparent clinical consequences treatment continuation may be appropriate.
If HP is persistent, severe or symptomatic in a patient with limited HAART options, phosphate replacement may be an option 2, and investigations to identify alternative causes of HP and associated renal dysfunction should be considered.
We stress the importance of post marketing surveillance to evaluate long term toxicities of new therapies.
|
|
| |
| |
 |
|
| |
| |
References
1. Fisher E, Chaloner K, Cohn D et al. The safety and efficacy of adefovir dipivoxil in patients with advanced HIV disease: a randomized, placebo-controlled trial. AIDS 2001;15:1695-1700.
2. Blick G, Greiger-Zanlungo P, Garton T et al. Tenofovir may cause severe hypophosphataemia in HIV/AIDS patients with prior adefovir-induced renal tubular acidosis. 10th Conference on Retroviruses and Opportunistic infections, Boston, USA 2003;abstract #718.
3. Reynes J, Peyriere H, Merle de Boever C et al. Renal tubular injury and severe hypophosphataemia (Fanconi Syndrome) associated with tenofovir therapy. 10th Conference on Retroviruses and Opportunistic infection, Boston,USA. 2003;abstract #717.
4. Verhelst D, Monge M, Meynard JL et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Diseases 2002;40:1331-1333.
5. Creput C, Gonzalez-Canali G, Hill G et al. Renal lesions in HIV-1-positive patients treated with tenofovir. AIDS 2003;17: 935-937.
6. Karras A, Lafaurie M, Legendre C et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome and nephrogenic diabetes insipidus. Clinical Infectious Diseases 2003;36:1070-1073.
7. Coca S and Perazella MA. Rapid communication: acute renal failure associated with tenofovir:evidence of drug-induced nephrotoxicity. Am J Med Sci 2002; 324:342-4.
8. Murphy M, O'Hearn M, Chou S. Fatal lactic acidosis and acute renal failure after addition of tenofovir to an antiretroviral regimen containing didanosine. Clinical Infectious Diseases 2003;36:1082-1085.
9. Gilead Sciences Inc. Background Package for NDA 21-356: VIREAD (tenofovir disoproxil fumarate).
10. Cheng A, Barriere S, Coakley F et al. Safety profile of Tenofovir DF in antiretroviral-experienced patients from randomised, double-blind, placebo-controlled clinical trials. XIV International AIDS Conference, Barcelona, Spain 2003;abstract TuPo4460.
11. Gilead Sciences Inc. Personal communication. February 2003.
12. Gallant.J et al. Similar 96-week renal safety profile of tenofovir DF versus stavudine (d4T) when used in combination with lamivudine and efavirenz in antiretroviral naïve patients. 43rd ICAAC, Chicago, USA 2003; abstract H-840.
13. Gilead Sciences. Viread Summary of Product Characteristics. 25th September 2003.
14. Miller J, Carr A, Emery S et al. HIV lipodystrophy: prevalence, severity and correlates of risk in Australia. HIV Med. 2003 Jul;4(3):293-301.
|
|
| |
|
 |
 |
|
|