 |
 |
 |
| |
IAS: 96 week results of Trizivir vs Combivir/Nelfinavir vs d4T/3TC/Nelfinavir: Trizivir story is not over yet
|
| |
| |
Written by Judith Aberg, MD, Washington University, St. Louis, Missouri and the ACTG
IAS: 96 week results of Trizivir vs Combivir/Nelfinavir vs d4T/3TC/Nelfinavir: Trizivir story is not over yet Written by Judith Aberg, MD, Washington University, St. Louis, Missouri and the ACTG
Comments by Jules Levin: At the IAS Conference, ACTG study 5095 found patients taking Trizivir (AZT, 3TC, and abacavir) experienced more virologic failure than patients taking efavirenz (Sustiva) combined with Combivir (AZT and 3TC). As a result the Data Safety and Monitoring Board stopped the study. When the study results were presented in an oral talk at IAS one of the authors of the study which is the subject of this article, Princy Kumar (Georgetown University), spoke at the open microphone and said that for some individuals Trizivir is a good treatment option. She was apparently referring to the results found in her study and described below. Trizivir is one pill taken twice a day, every 12 hours. It is relatively tolerable with fewer side effects, has a favorable lipid profile, is convenient to take, but as found in study 5095, it is not as potent as Sustiva. It is probably not as potent as Kaletra either. In the new PHHS HIV Treatment Guidelines, use of Trizivir for patients with >100,000 copies/ml is not recommended, because it is less potent.
In Dr Aberg’s report below she raises interesting questions regarding potential differences in response to HAART by gender and race; differences that may occur not only in viral response but lipid responses (cholesterol, triglycerides, and glucose). The study results and Dr Aberg’s comments suggest tolerability and convenience contribute to response to HAART, and there may be individual and perhaps cultural and ethnic differences that contribute to the success or failure of therapy. We all know by now that each HIV drug has its own set of unique side effects or potential toxicities. These study results suggest that perhaps gender, ethnicity, and race may play a more significant role in the success of therapy then we may have previously thought.
Excerpts from the author of this article, Dr Aberg: “…in light of the results of ACTG 5095 demonstrating the inferiority of TZV compared with EFV-containing regimens. …….These results indicate that therapy with Trizivir provides a more favorable lipid profile with comparable virologic and immunologic efficacy compared to these nelfinavir-containing regimens…… I think the question remains whether a subpopulation exists that may benefit from being on TZV alone…. but we all make treatment decisions based upon lifestyle, tolerance, adherence and complications of the regimen. Our ultimate goal is doing what is best for the individual”.
Oral 57. GENDER AND RACE SUBGROUP ANALYSES IN A PROSPECTIVE STUDY OF HYPERLIPIDAEMIA IN ART-NAIVE SUBJECTS TAKING TRIZIVIR (TZV), COMBIVIR (COM)/NELFINAVIR (NFV), OR STAVUDINE (D4T)/LAMIVUDINE (3TC)/NFV
K Tashima1, P Kumar2, A Rodriguez-French3, M Thompson4, P Wannamaker5, V Williams5 and K Pappa5 1 Miriam Hospital, Providence, RI, USA; 2 Georgetown University Medical Center, Washington, DC, USA; 3 San Fernando Hospital, Panama City, Panama; 4 ARCA, Atlanta, GA, USA; and 5 GlaxoSmithKline, RTP, NC, USA
Abstract 709. PROSPECTIVE STUDY OF HYPERLIPIDAEMIA IN ART-NAIVE SUBJECTS TAKING TRIZIVIR (TZV), COMBIVIR (COM)/NELFINAVIR (NFV), OR STAVUDINE (D4T)/LAMIVUDINE (3TC)/NFV
P Kumar1, A Rodriguez-French2, M Thompson3, K Tashima4, P Wannamaker5, V Williams5 and K Pappa5 1 Georgetown University Medical Center, Washington, DC, USA; 2 San Fernando Hospital, Panama City, Panama; 3 ARCA, Atlanta, GA, USA; 4 The Miriam Hospital, Providence, RI, USA; and 5 GlaxoSmithKline, RTP, NC, USA
I have previously reported on the 48-week data of this prospective study of hyperlipidemia in ART-naïve subjects taking Combivir/Abacavir (Trizivir, TZV) vs CBV/Nelfinavir (NFV) vs d4T/3TC/NFV presented by Dr. Kumar at the 9th Conference of Retroviruses and Opportunistic Infections (CROI) 2002, Abstract 33 (NATAP HIV and Cardiovascular Disease: True or Unrelated? The Debate Continues). There were two interesting observations in this study. One was that the d4t containing arms had a significant increase in LDL, TC, TG compared with CBV/NFV and TZV. The TZV arm had minimal changes in the lipid profiles. The other major observation was that men had a more significant increase in their LDL levels in both the NFV containing arms. At this IAS meeting, Dr. Kumar (poster abstract 709) now presents the 96 week results and Dr. Karen Tashima (oral abstract 57) presents the gender and race subgroup analysis.
A gender and racially diverse population was enrolled in this study which offers a unique opportunity to compare metabolic complication and response rates in these populations. The objectives of this study were to evaluate change in LDL and other lipid parameters as well as the safety and efficacy in subjects treated with TZV (Trizivir: AZT + 3TC +abacavir) vs CBV/NFV vs d4T/3TC/NFV (groups 1,2 and 3). Subgroup evaluations were assessed at 96 weeks. CBV or Combivir is AZT and 3Tc co-formulated in the same pill.
A total of 254 non-diabetic, ART-naive subjects from the US, PR, Panama, Dominican Republic and Guatemala with CD4 >50 cells/mm3 were randomized 1:1:1 to receive therapy as described above. An intensification regimen was allowed for subjects whose HIV VL remained >400 but <2,000 copies/ml and for those who had viral rebound to those parameters after achieving VL suppression to <400 copies/ml. The intensification consisted of adding efavirenz (EFV) to the TZV arm or abacaavir (ABC) to the NFV-containing arms. Evaluations consisted of fasting lipid profiles, HIV-1 RNA and CD4+ cell counts. Results were assessed in an analysis of covariance model. Statistical significance (ss) defined as P≤0.05.
In this diverse population (50% female, 40% black, 37% Hispanic, 21% Caucasian), the groups were comparable at baseline with overall mean age of 35 years, HIV RNA 4.43 log10 c/ml (27,000 copies/ml) and CD4+ 355 cells/mm3. Unfortunately the drop out rate or loss to follow-up was 49%. The withdrawal rate was similar for gender (53% female) and slightly lower in subjects from Latin America compared to the USA.
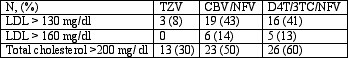
Overall, LDL, total cholesterol and triglycerides (TG) were significantly lower in the TZV alone arm compared with the NFV-containing arms. Again, they demonstrated the increase in TG in the d4T-NFV arm relative to the AZT-NFV arm. Significantly more subjects in the NFV containing arms had lipids above the recommended NCEP Guidelines. HDL was not significantly different among the 3 arms. See table below.
|
|
| |
| |
 |
|
| |
| |
Dr. Tashima described the metabolic subgroup analysis. Mean LDL was significantly lower in group 1 compared to groups 2 and 3 for men (93, 117, 126 mg/dl, groups 1, 2, 3; P=0.012 vs group 2; P<0.001 vs group 3) and women (94, 121, 134 mg/dl, groups 1, 2, 3; P<0.001 vs groups 2 and 3). Similar responses were seen for blacks (104, 120, 142, groups 1, 2, 3; ss: 1 and 2 vs 3) and hispanics (89, 111, 111 mg/dl, groups 1, 2, 3; ss: 1 vs 2 and 3). Triglycerides were significantly higher in hispanics in group 3 (188, 211, 295 mg/dl, groups 1, 2, 3; ss: 1 and 2 vs 3). Total cholesterol was significantly higher in group 3 for females (173, 201, 226 mg/dl, groups 1, 2, 3; ss: 1 vs 2 and 3) and hispanics (162, 193, 201 mg/dl, groups 1, 2, 3; ss: 1 vs 2 and 3). Elevated lactate with d4T was seen in female and hispanic subgroups (female ss: 1 vs 3; hispanic ss: 1 vs 2 and 3).
Dr. Tashima concluded that both metabolic and virologic responses to the different regimens appear to vary by gender and race and that further study is warranted. I spoke with Dr. Kumar and e-mailed Dr. Tashima afterwards and asked about differences in gender by race but given the high discontinuation rate, there isn’t an adequate sample size to answer these questions. I think this is an important issue that needs to be addressed. Would a black female be at the same risk of developing lipid abnormalities as a Hispanic male or Hispanic female or black man?
I also think this is important because not only do we see differences among the lipid parameters by race and gender, we also see different virologic responses suggesting that there may be significant differences genetically among us in regards to drug metabolism pathways or genetic variation in host lipoprotein pathways. The HIV virologic response (ITT Observed/ M=F) was similar across groups with proportions <400 copies/ml (ITT observed/ M=F) of 91%/48%, 89%/48% and 88%/44% for groups 1, 2 and 3. Mean CD4+T-cell increase at 96 wks was 265, 270 and 289 cells/mm3 for groups 1, 2 and 3. The mean absolute CD4+ T-cell count at 96 weeks was 619, 625 and 643 cells/mm3 for groups 1, 2 and 3 respectively. The proportion of subjects with HIV VL <50 copies/ml (ITT/observed on treatment) were 41%/78%, 39%/72% and 33%/66%) for groups 1, 2 and 3. In those subjects with HIV VL > 100,000 copies/ml at entry, the proportions of subjects achieving HIV VL <50 copies/ml (ITT, M=F) showed a similar pattern of response with 53%(TZV), 42% (CBV/NFV), and 29% (d4T/3TC/NFV). Women tended to have less virologic response compared with men. In addition, response rate for TZV was consistently higher in hispanics (HIV RNA <400 copies/ml, 65%, 45%, 44%; HIV RNA <50 copies/ml, 61%, 39%, 31% for groups 1, 2, 3). Among those with virologic failures who had genotypes available from baseline and at time of failure, M184V was identified in 1 out of 4, 6 out of 10 and 5 out of 6 in groups 1,2 and 3 respectively. The D30N or L90M was selected in 44% of subjects failing in the NFV-containing arms. Common drug-related Grade 2–4 AEs (>5%) were diarrhea, nausea, abdominal pain, vomiting, fatigue, and neutropenia as seen in similar trials.
So, in light of the major news of the conference being the results of ACTG 5095 demonstrating the inferiority of TZV compared with EFV-containing regimens, this study suggests the long term efficacy of TZV is similar to the NFV-containing regimens with a trend favoring the TZV alone arm. These results indicate that therapy with TZV provides a more favorable lipid profile with comparable virologic and immunologic efficacy compared to these NFV-containing regimens. I think the question remains whether a subpopulation exists that may benefit from being on TZV alone. I agree with the study team that further studies are warranted to evaluate whether treatment responses and complications are significantly different with respect to gender, race and ethnicity so that therapies can be prescribed to achieve the maximum benefit for that individual. I think given the results of ACTG 5095 and CNA 3005 (Tzv vs IDV-containing regimen), it is unlikely that another study using a TZV alone arm will be conducted given the concerns of prescribing a potentially inferior regimen but I do believe there may be a subpopulation who would benefit from this simple regimen and one should not necessarily say this should never be prescribed. It may not be the optimal regimen but we all make treatment decisions based upon lifestyle, tolerance, adherence and complications of the regimen. Our ultimate goal is doing what is best for the individual.
|
|
| |
|
 |
 |
|
|