 |
 |
 |
| |
Does Early HCV Kinetics Predict Response to Peginterferon and Ribavirin HCV Therapy among HIV Co-infected Individuals
Reported by Jules Levin
|
| |
| |
Martin Pols from the NIH National Institute of Diabetes, Digestive, and Kidney Diseases reported this small preliminary pilot study at the Intl AIDS Conference in Paris at an oral session.
Pols examined the 12 week stopping rule in co-infected patients now generally accepted in treating HCV monotherapy. Studies have found that if a patient does not reduce HCV viral load by 2 log by week 12 after starting peginterferon plus ribavirin they are very unlikely to achieve a sustained viral response. So unless there are other reasons to continue therapy, such as for maintenance therapy, the patient should consider stopping therapy at week 12. In this pilot study by NIDDK, the data suggests to Pols that the slope of HCV viral load decline, not necessarily the 12-week viral load response, appears to predict EVR, ETR, for HCV in HCV/HIV co-infected patients. Pols says the predictive value for SVR in this population has yet to be determined. Clearly, the data from this study is based on too small a number of patients and is preliminary; but hopefully this study will be followed up with a larger study to examine these findings. Pols also says that the data from this study suggests, as other studies have also suggested, that HCV/HIV co-infected patients may respond slower to HCV therapy than persons infected with HCV alone. Pols concludes that these findings underscores the suggestion that the 12-week stop rule may not work for co-infected patients.
Few models are known for predicting HCV response in co-infected patients based on early therapy results. The purpose of this study is to examine the ability of early HCV viral kinetics to predict early virologic response (EVR) and end of treatment response (ETR). And to determine the safety and efficacy of a combination therapy with peginterferon a-2b and ribavirin among a co-infected population.
Patients were excluded from this study for having decompensated cirrhosis or for current alcohol or substance abuse. ART was used at the discretion of the patient and referring physician.
Patients received weekly sc Peg-IFN a-2b (1.5ug/kg) with oral ribavirin (RBV) 1000 to 1200 mg/day, in divided doses. Therapy is for 48 weeks. Erythropoietin (procrit) was used to support hemoglobin <10 g/dL and CSF for <750 PMN's/cu mm. A liver biopsy was performed prior to and after completion of therapy.
HCV VL, HIV VL, CD4, and CD8 counts were measured prior to the beginning of therapy, on days 1, 3, 5, 7, and 10, and then every 4 weeks thereafter.
Early Virologic Response (EVR) was defined as a decrease in HCV viral load in plasma of 2 logs or more by week 12 on therapy.
End of Treatment Response (ETR) was defined as the absence of detectable HCV in plasma at the completion of therapy.
Sustained Virologic Response (SVR) is defined as the absence of detectable HCV in plasma 24 weeks after the completion of therapy.
RESULTS
--29 patients have enrolled
--27 male, 2 female, 16 African-American, 12 Caucasian, a Hispanic
--25 HCV genotype 1, 3 genotype 2, 1 genotype 3
--13 subjects had HCV viral load >2 million
--24 subjects on HAART, 5 not on ARVs
--20 subjects HIV VL <50 copies/ml, 8 had HIV VL ranging from 71 to 33,000 copies/ml
--CD4 counts:
1 100-200
2 200-300
5 300-400
3 400-500
18 >500
--29 patients who have received therapy for up to 48 weeks (23 of whom have completed at least 12 weeks) with a median of 36 weeks, ranging from 5 days to 48 weeks
--18 individuals have completed therapy:
11 completed 48 weeks of therapy
7 individuals have stopped therapy before 48 weeks
-3 for viral non-response
-2 for toxicities (color vision loss, fatigue)
-2 for social reasons
--11 patients remain on therapy
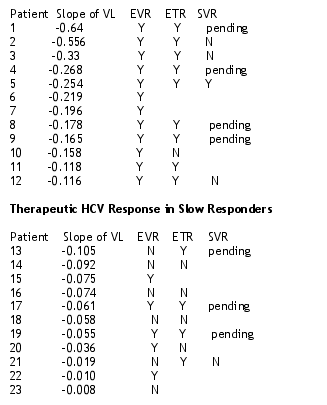
Therapeutic HCV Response in Early Viral Responders
As you can see of the 9 patients for whom they have data on at end of treatment, 8 whom were early responders at week 12 had an ETR. But 3 of these 8 did not have a SVR. Results on 4 patients are pending. It appears that 3 patients may have discontinued after 12 weeks.
|
|
| |
 |
|
| |
12/12 (100%) of persons with an HCV viral load slope (day 0-10) > .11 had an EVR:
--8/9 (89%) completing therapy have had an ETR
--1/4 (25%) of persons with an ETR completing 24 weeks after therapy have had an SVR
5/11 (45%) of persons with a HCV viral load slope (day 0-10) <.11 had an EVR:
--4/8 (50%) of persons completing therapy have had an ETR
--0/1 (0%) of personw with an ETR completing 24 weeks after therapy have had an SVR
|
|
| |
| |
|
 |
 |
|
|