 |
 |
 |
| |
Anti-Hepatitis B Virus (HBV) Activity of Tenofovir Disoproxil Fumarate (TDF) in Human Immunodeficiency Virus (HIV) Co-Infected Patients
|
| |
| |
43rd ICAAC, Sept 2003, Chicago. Poster 2467
P. TRIMOULET (1), V. THIBAULT (2), V. SCHNEIDER (3), I. PELLEGRIN (1), L. BOCKET (4), M. P. SCHMITT (5), C. POGGI (6), D. VITTECOCQ (7), C. PIKETTY (8), Y. BENHAMOU (2).
1 Hopital Pellegrin, Bordeaux, 2 Groupe Hospitalier Pitie Salpetriere, Paris, 3 Hopital Tenon, Paris, 4 Hopital de Tourcoing, Tourcoing, 5 Hopital Universitaire, Srasbourg, 6 Hopital Chalucet, Toulon, 7 Hopital Paul Brousse, Villejuif, 8 Hopital Georges Pompidou, Paris. France.
Summary: In this retrospective study researchers reported on 109 HIV+ and HBV+ patients at 18 HIV clinics in France who added tenofovir to their HIV regimen to evaluate the effect on HBV viral load. They looked at stored blood samples of the patients to measure the reduction in HBV viral load after adding tenofovir. 96% of the patients were on 3TC, and 85% of patients on 3TC had detectable HBV DNA. After following patients for a median of 9 months the HBV viral load (HBV-DNA) reduction was –3.8 log, and 33% of patients became undetectable (<2.3 log).
The following report contains excerpts from the poster at ICAAC.
ABSTRACT
Pilot studies have suggested TDF efficacy against HBV in HIV infected patients (Pts). Anti-HBV efficacy of TDF (300 mg.qd), administered as a part of antiretroviral therapy, was analyzed in a large cohort of HIV/HBV co-infected Pts.
HIV/HBV co-infected Pts who had received TDF for at least 2 weeks and who had stored serum samples at initiation and during TDF therapy were included. Serum HBV DNA was measured on stored samples by PCR (sensitivity 2.3 log10copies/mL). Baseline was the day of TDF initiation and the end point was the day of the last available stored serum sample during TDF therapy.
A total of 109 Pts (mean age 42.6 ± 7.0 years) were included. The median follow-up period was 8 (1 - 24) months. All the Pts but 7 (96.2%) were receiving lamivudine (150 mg bid) at baseline. Median CD4 count and HIV RNA at baseline were 350 (3 - 1174) cells/µL and 2.6 (1.3 - 6.1) log10copies/mL, respectively. Twenty six Pts (26.3%) had an HIV RNA <50 copies/mL. HBV DNA was detectable at baseline in 90 Pts (84.1%). 83 (95.4%) among them were receiving lamivudine at TDF initiation. After a median period of 10.2 (2-24) months HBV DNA became undetectable in 30 (33.7%) Pts. In Pts with detectable DNA at baseline the median change from baseline of serum HBV DNA (8.0, 2.3 - 8.8) log10copies/mL) was –3.8 (–6.4 - –2.5) log10copies/mL (p<0.0001).
The preliminary results of this large study demonstrate the anti-HBV efficacy of TDF (300 mg.qd), given for a median period of 8 months, in HIV infected Pts with HBV replication despite lamivudine therapy.
INTRODUCTION
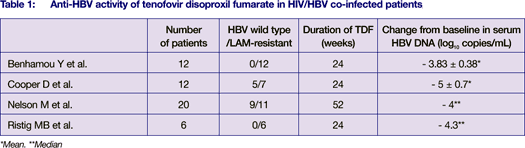
Chronic infection with hepatitis B virus (HBV) affects over 400 million people worldwide, about 5% of the world population. Among human immunodeficiency virus (HIV) infected individuals, the prevalence of HBV infection is approximately 10 fold higher and is associated with a higher risk of cirrhosis and death. Lamivudine inhibits HBV replication in more than 80% of cases in both HIV and non-HIV-infected CHB patients. However, emergence of HBV resistance to lamivudine (LAM-R) has been widely observed. HBV resistance to lamivudine has been reported with an incidence of 50% and 90% after 2 and 4 years of therapy, respectively. The clinical consequences of HBV resistance in HIV-positive patients are unknown. However, as observed in HBV mono-infected patients, cases of CHB exacerbation and liver failure have been reported in HIV/LAM-R HBV co-infected individuals. (exacerbations of HBV have been reported in patients after the discontinuation of TDF. Patients co-infected with HIV & HBV should be closely monitored with both clinical and lab follow-up for at least several months after stopping lamivudine or TDF). Until recently, there has not been an effective treatment for LAM-Resistant HBV. We previously reported the safety and efficacy results of 48 weeks of ADV in co-infected patients with LAM-R HBV. Mean serum HBV DNA decreased from baseline (8.64 log10 copies/mL) by –4.07 log10 copies/mL at week 48 associated with improvement of liver lesions and ALT normalization.
Tenofovir disoproxil fumarate (TDF) is approved for treatment of HIV-1 infection as a once daily 300 mg tablet and has recently been shown to have significant activity against both HIV and HBV. TDF is a nucleotide reverse transcriptase inhibitor and has been shown to have potent in vitro activity against both wild type and lamivudine resistant HBV. In short term small pilot studies, TDF demonstrated anti HBV activity in HIV/HBV co-infected patients.
|
|
| |
| |
 |
|
| |
| |
However, a larger patient population and a longer treatment period are necessary to assess the extent and durability of HBV suppression, as well as its long term tolerance profile, the potential emergence of resistance to TDF and the HbeAg seroconversion rates.
We retrospectively analyzed in real life efficacy and safety of TDF on HBV replication in HIV-infected patients.
Patients Patients were recruited in 18 Infectious Diseases Department in France from January 2001 to January 2003. Patients with HBV and HIV co-infection were included in this study if they: --Received TDF for at least 2 weeks --Had stored serum at TDF initiation and during TDF therapy
Serum HBV DNA was measured by a polymerase chain reaction (PCR) assay with a 2.3 log10 copies/mL sensitivity (Amplicor, HBV Monitor Cobas, Roche, Meylan, FRANCE).
RESULTS
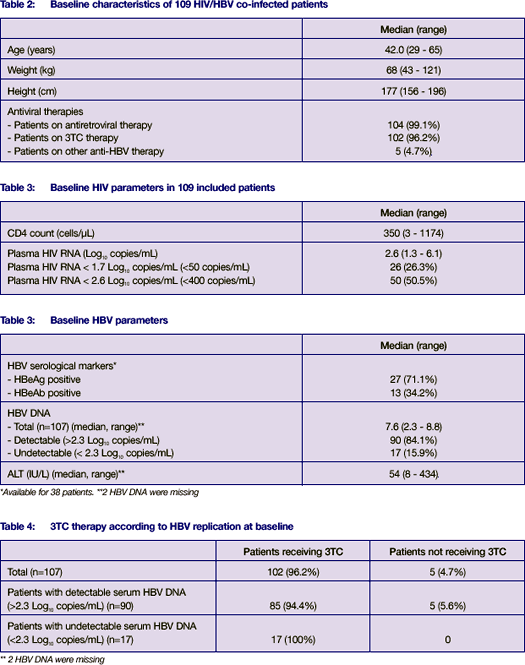
A total of 109 patients with HIV and HBV were included in the study. The main baseline characteristics are summarized in tables 2 to 4. The median follow-up was 8 months (1 - 24).
|
|
| |
| |
 |
|
| |
| |
Among the patients with detectable serum HBV DNA at baseline; 33.7% of patients became undetectable (serum HBV DNA < 2.3 log10 copies/ml).
All the patients with undetectable serum HBV DNA (< 2.3 log10 copies/ml) at baseline remained undetectable after a median period of TDF of 5.0 (1-17) months.
AUTHORS CONCLUDED
The preliminary results of this first large cohort study strongly suggest the efficacy of Tenofovir Disoproxil Fumarate for the control of HBV replication in HIV co-infected patients. The median reduction of serum HBV DNA was 3.8 Log10 copies/mL after a median follow-up of 9 months of treatment. No TDF-related serious adverse event was recorded. Tenofovir Disoproxil Fumarate was active against wild type HBV, presumed pre-core mutant HBV and presumed lamivudine resistant HBV. Serum HBV DNA follow-up, HBV serological makers and HBV genotyping tests are ongoing
|
|
| |
|
 |
 |
|
|