 |
 |
 |
| |
|
PK Drug Interaction and Safety of Tenofovir and Kaletra
|
| |
| |
Reported by Jules Levin
ICAAC poster A-1617. Kearney et al, Gilead Sciences. ICAAC Sept 14-17, 2003, Chicago.
The goal of this PK study was to evaluate the effect of TDF and Kaletra (LPV/r) co-administration on the PK and safety of TDF, LPV, and RTV. And to evaluate the long term safety of concomitant use of TDF and LPV/r in HAART regimens of heavily treatment experienced patients with advanced HIV.
A previous drug-drug interaction study identified small changes in the PK of TDF, lopinavir, and RTV during their co-administration that were not considered clinically relevant and similar LPV/RTV PK has been reported in HAART regimens with or without TDF.
Study drugs were administered within 5 minutes of completion of a standardized light meal (about 373 kcal, 20% fat). 27 HIV-negative individuals received TDF 300 mg QD with a light meal for 7 days. Then subjects were randomized to with or without LPV/r 400/100 (co-administered following a moderate fat meal) plus TDF for 14 days. For another 14 days patients were crossed over to the other arm.
In order to look at the clinical significance of using the combination, patients in a compassionate access study (GS-908) receiving TDF+Kaletra were assessed for evidence of clinically significant nephrotoxicity through analysis confirmed changes in serum creatinine (>2.0 mg/dl) and phosphorous (<1.5 mg/dl) using a centralized lab.
RESULTS
PK of Lopinavir alone and RTV alone were unaffected by TDF.
Steady state TDF exposures were increased by 32% when TDF was coadministered with LPV/r, and this was similar to that observed in previous study.
|
|
| |
| |
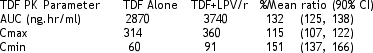 |
|
| |
| |
Safety Results of PK Study
The PK study was for 35 days. The authors reported the following. No serious adeverse events were reported. 23 (85%) of subjects experienced at least 1 treatment-emergent AE. Most common AE was diarrhea (67% of subjects reported at least 1 episode): --TDF alone (n=4); LPV/r alone (n=13); TDF+LPV/r (n=14) --2 subjects withdrew consent due to diarrhea --other frequent adverse events were: headache (52%), nausea (32%), and abdominal pain (25%) --one subject experienced elevated liver function tests atributed to LPV/r --one subject experienced tachycardia attributed to both TDF and LPV/r.
Study GS-908 TDF Compassionate Access Study (n=296)
LPV/r was used in 271/296 (94%) of patients. Mean duration of concomitant use was 63 weeks (maximum 96 wks). Mean HIV RNA 4.9 log; mean CD4 count 36; prior AIDS diagnosis 93%; mean age 42; 93% male; race: 70% caucasian; 16% AA; 12% Hispanic; 2% Asian.
The incidence of confirmed changes in serum creatinine to >2.0 mg/dl or serum phospohorous <1.5 mg/dl was <1%.
Five patients (1.8%; 5/271) experienced serum creatinine changes leading to TDF discontinuation: one patient developed Fanconi’s Syndrome (also experienced during prior use of high dose adefovir).
In sum, TDF levels increased when used with Kaletra in this study of short duration. Further studies should be conducted with long-term follow-up controlling for patients with renal dysfunction to address this question more comprehensively. Regular monitoring of kidney/renal functioning for patients on this regimen should be helpful.
|
|
| |
|
 |
 |
|
|