 |
 |
 |
| |
The Ongoing Story of Kaletra Resistance
|
| |
| |
"Comparative Incidence and Temporal Accumulation of PI and NRTI Resistance in HIV-Infected Subjects Receiving Lopinavir/ritonavir or Nelfinavir as Initial Therapy"
Kempf et al. poster #600, 10th Conference on Retroviruses and Opportunistic infections, Boston, MA, Feb 2003
A brief summary of this study by Jules Levin: (1) After following patients now for 96 weeks in the pivotol study (for FDA approval) by Abbott comparing Kaletra vs nelfinavir (all patients also received d4T/3TC), no resistance has been observed to Kaletra (0/51) among the 51 patients for whom genotype tests were available. For the 96 patients who took nelfinavir and had genotype tests available, 48% (46/96) had nelfinavir resistance.
(2) When there is ongoing viral replication (viral load >400 copies/ml) resistance to 3TC in this study develops more quickly and more often for patients on nelfinavir compared to patients on Kaletra. This study explores these questions and elaborates on the data the study authors found. ItŐs quite interesting that Kaletra resistance has not been observed and that 3TC resistance develops more slowly and less often in patients on Kaletra.
(3) Resistance to d4T was relatively low: after 6 months of continuously detectable viral load following rebound, 7% of patients on nelfinavir had d4T resistance; and after 1 year 15% had d4T resistance. For patients on Kaletra d4T resistance occurred less often.
These findings should be considered in selecting an initial regimen for treatment of HIV. See additional report relevant to this study, which include comments in the NATAP Resistance Report from Retrovirus written by Andrew Zolopa, MD: http://www.natap.org/2003/Retro/day28.htm
The Study Report:
The emergence of resistance to different drugs during combination antiretroviral (ARV) therapy may occur at substantially different rates. These
differences are best estimated by systematic evaluation of the incidence of resistance in comparative clinical studies. Study M98-863 was a
multi-center, multinational, blinded, randomized, prospective study that compared the antiviral activity and safety of lopinavir/ritonavir (LPV/r) plus d4T and 3TC to that of nelfinavir (NFV) plus d4T and 3TC in ARV-naive subjects. A total of 653 subjects were enrolled in the study (LPV/r n=326,
NFV n=327). Through Week 60, significantly more LPV/r-treated subjects experienced viral suppression to HIV RNA levels less than 50 copies/mL than
NFV-treated subjects (64% vs. 53%, respectively, intent to treat analysis).
1 Previously, the incidence of protease inhibitor (PI) and 3TC resistance
among subjects with HIV RNA >400 copies/mL and available genotype on therapy has been analyzed. The updated results, based on a
complete data set, are provided in Table 1.
|
|
 |
| |
In the current analysis (poster #600), we assessed the temporal emergence of PI and NRTI resistance, both from the initiation of therapy and the point of viral rebound, using a Kaplan-Meier approach. By accounting for subjects who discontinued study medications, the Kaplan-Meier method allows for
unbiased estimates of the cumulative resistance to each individual drug in the ARV regimen. Such estimates can provide probabalistic information for
the risk/benefit assessment of various combination regimens used for initial ARV therapy.
Samples were selected for genotypic testing at Weeks 24, 48, and 60 for subjects with HIV RNA above 400 copies/mL at those visits, as well as at
the final study visit (Week 72, 84, or 96) with HIV RNA >400 copies/mL. For subjects without a detectable viral load at Week 48, a sample from
Weeks 32-40 was sent for genotypic testing if HIV RNA was >400 copies/mL.
Definition of Resistance
Subjects with continuous HIV RNA <400 copies/mL between Weeks 24 and 96, and subjects with HIV RNA >400 copies/mL but without available genotype were considered as not displaying resistance. For LPV/r, PI resistance was defined as the emergence of any primary or active site mutation in protease (amino acids 8, 30, 32, 46, 47, 48, 50, 82, 84 or 90). The absence of resistance was confirmed by phenotypic analysis for all samples from LPV/r-treated subjects. For NFV, PI resistance was defined as the emergence of the D30N or L90M mutation in protease, or the emergence of the M46I/L mutation in protease with confirmed reduced phenotypic susceptibility to NFV. 3TC resistance was defined as the emergence of the M184V/I/T mutation in reverse transcriptase. Resistance to d4T was defined as the emergence of any thymidine-associated mutation in reverse transcriptase (M41L, D67N, K70R, L210W, T215F/Y and K219Q/E/N). One subject whose genotype clearly suggested the re-emergence of a pre-existing resistant mutant (simultaneous appearance of mutations at positions 67, 70, 215 and 219 at the first time point with viral load >400 copies/mL and an apparent archival sequence [T/A/I/V] at position 215) was not considered to acquire d4T resistance during therapy.
Secondary protease mutations were defined as any change at the following codons: position 10, 20, 24, 33, 36, 46, 54, 71, 73, 77 or 88.
Subjects who never achieved HIV RNA <400 copies/mL were excluded from the analysis. Subjects who did not demonstrate resistance were censored as of their final available genotype after viral load rebound.
RESULTS
Analysis of the Rates of Resistance Development from the Initiation of Therapy Through Two Years
In order to assess the risk of resistance development through two years of therapy, the overall rates of PI resistance, 3TC resistance and d4T resistance for each treatment group in this study were estimated using Kaplan-Meier analysis.
The analysis provided the following estimates for resistance emergence among all treated subjects over two years following initiation of therapy.
NFV and d4T resistance were always accompanied by 3TC resistance.
- 3TC resistance (NFV group): 29%
- PI + 3TC resistance (NFV group): 20%
- 3TC resistance (LPV/r group): 7%
- d4T + 3TC resistance (NFV group): 5%
- PI or d4T resistance (LPV/r group): 0%
All pairwise comparisons of the above groups were statistically significant at p< 0.001 except for the comparison of 3TC resistance in the LPV/r group and d4T resistance in the NFV group (p=0.09).
The onset of d4T resistance in NFV-treated subjects was less rapid than that of 3TC resistance in LPV/r-treated subjects. However, the resistance rates were comparable after two years, potentially due to ongoing viral replication in many of the NFV-treated subjects.
The majority (8/9) of subjects with d4T resistance also demonstrated NFV resistance. The overall rate of development of triple-drug resistance in
NFV-treated subjects based on Kaplan-Meier analysis was 4.5%.
The large incremental increases in resistance observed at Weeks 24 and 48 are likely a result of the timing of sample selection, since those samples were submitted for genotype first and no samples were analyzed prior to Week 24.
Analysis of the Rates of Resistance Development During Detectable Viral Load
In order to further assess resistance emergence, a second analysis of resistance rates during periods of detectable viral load was performed.
A total of 197 subjects (123 NFV, 74 LPV/r) had viral load above 400 copies/mL at or after Week 24.
Genotype data were available for 51/74 (69%) LPV/r-treated subjects and 96/123 (78%) NFV-treated subjects with detectable HIV RNA.
Genotype data were not available for 50 subjects (27 NFV, 23 LPV/r), in general due to low viral copy numbers. The median (interquartile range,
IQR) viral load in copies/mL was 819 (477-1693) for subjects without genotype data and 3365 (1052-17444) for subjects with genotype data (p<0.001, Wilcoxon rank-sum test). Viral load in the majority of these subjects (LPV/r: 17/17; NFV: 18/24) for whom subsequent HIV RNA values were available subsequently re-suppressed to <400 copies/mL without change in treatment regimen.
A minority of subjects with genotype (21/147, 14%) never experienced HIV RNA suppression to <400 copies/mL. Genotype was available for 19/21
of these subjects at the Week 24 time point and 2/21 at the Week 48 time point.
The majority of subjects with genotype (126/147, 86%) demonstrated initial HIV RNA decline to <400 copies/mL followed by viral rebound. The initial
genotype was available at the first rebound time point for 64 (51%) of these subjects (LPV/r: n=30, NFV: n=34).
The remaining 62 subjects with genotype had one or more time points with detectable viral load prior to the genotype sample. The median (IQR)
duration since the first HIV RNA >400 copies/mL for this group was 8 (5-13) weeks.
Temporal Accumulation of PI and NRTI Resistance
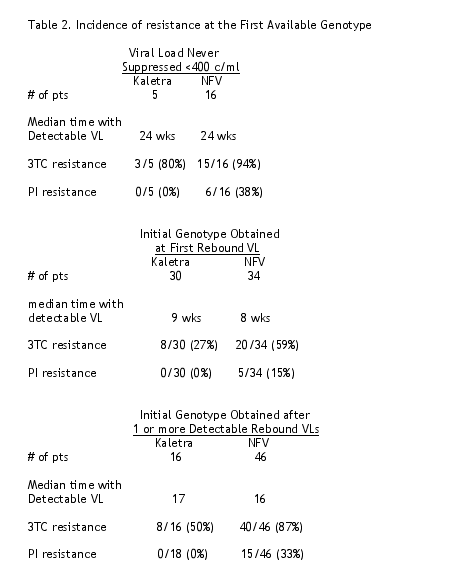
- Among all 147 subjects with available genotype, resistance observed in the initial genotype is summarized in Table 2.
- 3TC resistance was evident at the first available genotype in 94/98 subjects who ultimately developed 3TC resistance.
- NFV resistance was evident at the first available genotype in 26/46 NFV-treated subjects who ultimately developed PI resistance.
- Two or more longitudinal sequences were available from 47 NFV-treated subjects who did not demonstrate NFV resistance at first genotype.
Subsequent development of NFV resistance was observed in 20/47 subjects.
- NFV resistance emerged prior to the appearance of d4T resistance in 4/8 subjects who demonstrated resistance to all three drugs in the regimen.
|
|
 |
| |
Kaplan-Meier Analysis of Resistance Development During Detectable Viral Load
- Kaplan-Meier analysis was performed on the subset of 126 subjects with viral suppression to <400 copies/mL followed by viral rebound and available genotype. In this analysis, time zero was defined as the last study visit at which HIV RNA was <400 copies/mL.
- Subjects with ongoing viral replication during treatment with NFV/3TC/d4T were at high risk of developing resistance.
- After 6 months of continuously detectable viral load following viral rebound, 3TC, PI, and d4T resistance rates were 86%, 42%, and 7%, respectively.
- After 1 year of continuously detectable viral load following viral rebound, 3TC, PI, and d4T resistance rates were 100%, 74%,
and 15%, respectively.
- The temporal emergence of resistance to 3TC between the two study arms is compared.
- Among subjects with viral rebound after viral suppression <400 copies/mL, 3TC resistance emerged significantly more rapidly among NFV-treated
subjects compared to LPV/r-treated subjects (p<0.002, log-rank test).
- After 6 months of continuously detectable viral load following viral rebound, 3TC resistance rates were 86% for the NFV group and 48% for the LPV/r group.
Temporal Emergence of Secondary Protease Mutations in LPV/r-treated Subjects
- PI resistance did not emerge in LPV/r-treated subjects in this study. However, in 7/51 subjects with genotype, a new secondary protease mutation
was observed at position 36, 10 or 71.
These genotypic changes had no effect on the phenotypic susceptibilities of the isolates to LPV.
- Only 4/7 subjects with a new secondary PI mutation demonstrated 3TC resistance.
- The exposure to viral replication in the seven subjects was comparable to that in the remaining 44 LPV/r-treated subjects with no new secondary mutations.
- The longest continuous period of detectable viral load without resistance in a LPV/r-treated subject was 72 weeks.
CONCLUSIONS BY STUDY AUTHORS
- The incidence of resistance to each component of the regimen (PI, 3TC and d4T) was significantly lower in LPV/r-treated subjects than in NFV-treated subjects despite identical NRTIs in the two arms of this randomized comparative study.
- From most to least common, the relative rates of resistance development among all enrolled subjects were: 3TC resistance (NFV group) > PI +
3TC resistance (NFV group) > 3TC resistance (LPV/r group) ~ d4T + 3TC resistance (NFV group) d4T or PI resistance (LPV/r group).
- Among subjects demonstrating resistant virus, NFV resistance and 3TC resistance were frequently observed at the first available genotype.
- Persistent viral replication in subjects receiving NFV plus d4T/3TC was associated with a high risk of 3TC and NFV resistance. Persistent viral
replication in subjects receiving LPV/r plus d4T/3TC was associated with a moderate risk of 3TC resistance but a low risk of d4T or LPV resistance.
- The relatively low probability of resistance development demonstrated in the LPV/r arm of this study may have important implications for the choice
of initial ARV therapy.
|
|
| |
|
 |
 |
|
|