| |
Antiretrovirals and Cardiovascular Disease: Is HAART Bad for Your Heart?
|
| |
| |
AIDS Clinical Care, August 2003
By Carl J. Fichtenbaum, MD
A variety of cardiovascular problems, including cardiomyopathy, pulmonary hypertension, arrhythmias, and endocarditis, have been reported in the setting of HIV infection. The widespread use of potent combination antiretroviral therapy has resulted in a 60% to 80% decline in AIDS-related morbidity and mortality, with a resultant decrease in the frequency of many of these cardiovascular problems. Unfortunately, the use of such therapy has also been associated with an array of metabolic disorders. Documentation of dyslipidemia, insulin resistance, and visceral adiposity raises concerns about premature or accelerated development of atherosclerosis in antiretroviral-treated HIV-infected individuals. A number of case series and anecdotal reports of symptomatic coronary artery disease (CAD) in this population have been published in the past three years. In addition, as HIV-infected individuals live longer, they are increasingly affected by vascular diseases commonly associated with the aging process. Thus, HIV care providers should become familiar with the evidence of CAD in HIV-infected individuals, evaluating and managing risk factors for CAD, and treating the metabolic complications associated with potent combination antiretroviral therapy.
CARDIOVASCULAR DISEASE IN THE GENERAL POPULATION
Cardiovascular disease is the leading cause of death in males over the age of 35 and all persons over the age of 45 in the U.S. Atherosclerosis is a complex inflammatory process marked by the presence of lymphocytes, foamy macrophages, enhanced expression of adhesion molecules, proliferation of smooth muscle cells, and, ultimately, maturation of lipid-laden plaques. A number of well-described risk factors are associated with CAD, including a family history of premature atherosclerosis (symptomatic CAD in a man age <55 or a woman age <65), diabetes mellitus, hypertension, cigarette smoking, obesity, physical inactivity, and hyperlipidemia. Medical interventions have focused on altering modifiable risk factors such as lifestyle, diet, and cigarette smoking, while employing medical therapy to treat hypertension, hyperlipidemia, and diabetes mellitus. This approach, along with advances in the acute care of cardiac emergencies, has reduced CAD mortality over the past decade.
CARDIOVASCULAR DISEASE IN HIV INFECTION
CAD was infrequently described in association with HIV infection before the advent of potent combination antiretroviral therapy, perhaps because patients simply did not live long enough to develop CAD. Starting in the late 1990s, however, anecdotal reports of CAD fueled speculation that the metabolic disturbances associated with the use of potent combination antiretroviral therapy may accelerate the rate of progression of atherosclerosis. For example, Henry and colleagues reported the occurrence of symptomatic CAD in two young HIV-infected men. This was followed by several other reports of myocardial infarctions (MIs), severe CAD, or both, associated with the use of PIs. In addition, PI use has not been the only HIV-related factor implicated in CAD. David and colleagues reported a case-control study of 16 patients with proven coronary events suggesting that duration of NRTI use and CD4 nadir, but not PI use, were associated with the development of symptomatic CAD. None of these reports provides clear evidence of an association between the use of combination antiretroviral therapy and the occurrence of CAD. Indeed, Most of these cases occurred in men in their 40s or 50s with known CAD risk factors such as smoking or a family history of CAD.
EPIDEMIOLOGIC STUDIES OF CARDIOVASCULAR DISEASE IN HIV INFECTION
Several large, retrospective epidemiologic studies of CAD risk factors and events in HIV-infected individuals have been published or presented in the past year. Taken together, these studies indicate that increased risk for CAD is a genuine cause for concern in this population. However, these studies have several flaws: (1) none were designed to distinguish the effects of HIV infection from those of antiretroviral therapy, and the duration of HIV infection and the presence of immunologic disturbances or other coinfections may play a role in the development of atherosclerosis; (2) as with other retrospective studies, there was a potential ascertainment bias, particularly involving the inconsistent or nonstandardized collection of data regarding CAD risk factors; (3) atherosclerosis takes decades to develop, and even a 2-fold increase in the acceleration of the process may not manifest itself for 10 years, but most of these studies had a very short follow-up.
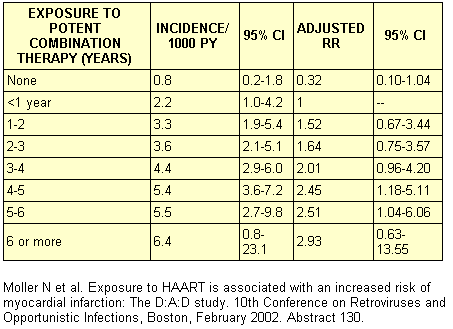
Bozzette and colleagues published a retrospective study of the prevalence of CAD events in 36,766 HIV-infected individuals treated at Veterans Affairs facilities. Between 1995 and 2001, the rate of admissions for CAD or cerebrovascular disease decreased from 1.7 to 0.9 per 100 patient-years. No association between the use of combination antiretroviral therapy and vascular events was noted. The limitations of this study include the passive reporting of events, omissions of data on known risk factors for CAD, and the short duration of combination therapy exposure (median, 16-17 months). The data collection on adverse events of anti-HIV drugs study (the DAD Study) is a prospective, multicohort study of 23,490 subjects based largely in Europe. The study has been powered to demonstrate a 2-fold increase in the risk for MI. During 36,479 patient-years of follow up, 129 MIs (45% definite, 55% possible) developed according to WHO criteria. Of these, 36 were fatal. The overall incidence of MI was 3.5 per 1000 patient-years. Potent combination antiretroviral therapy was associated with a 27% relative increase in the rate of MI per year of exposure over the first 7 years of use (see Table 1). Some weaknesses of this study were failure of some sites to obtain fasting lipid levels and the inclusion of many "possible" MI cases and many patients with prior CAD.
|
|
| |
| |
 |
|
| |
| |
Several lines of evidence -- including the presence of antiretroviral-associated dyslipidemia; insulin resistance; endothelial dysfunction; and abnormal carotid intima thickness; coronary artery calcifications, and elevated lipid and triglyceride levels -- suggest that atherosclerosis may be more frequent or develop more rapidly in HIV-infected individuals, particularly those receiving potent combination antiretroviral therapy.
Dyslipidemia is a frequent disturbance observed in treatment-naive HIV-infected individuals. The most common profile in untreated patients is an elevated triglyceride level and a decreased HDL choesterol level. Many antiretroviral agents induce changes in lipid profiles. Among the antiretroviral drug classes, PIs appear to have the most consistent effect on lipid levels. However, elevated cholesterol has been observed with the use of efavirenz and NRTIs such as d4T.
PIs are associated with dyslipidemia either directly through induction of cholesterol or fatty acid biosynthesis or indirectly through the development of insulin resistance. The most compelling evidence for the connection between PI use and dyslipidemia comes from studies in HIV-negative individuals demonstrating insulin resistance in association with short-term use of indinavir and hypertriglyceridemia associated with short-term use of ritonavir. In a study comparing 116 PI-treated patients with 45 PI-naive patients, Carr and colleagues reported elevated triglyceride (50% vs. 22%) and total cholesterol levels (58% vs. 11%) and combined hypertriglyceridemia/hypercholesterolemia (38% vs. 5%) in PI-exposed versus PI-naive individuals. Segerer reported a 15% increase in total cholesterol and a 25% increase in triglyceride levels from baseline after 3 to 6 months in subjects using saquinavir, ritonavir, nelfinavir, or indinavir. Behrens and colleagues reported that 71% of PI-treated subjects (n=27) had detectable hyperlipidemia. Triglyceride, LDL, very-low density lipoprotein (VLDL), and Apolipoprotein B and E levels were all significantly increased compared with PI-naive HIV-infected subjects. Stein demonstrated the presence of an atherogenic dyslipidemia in 37 adults receiving combination antiretroviral therapy. Those receiving PIs had higher levels of intermediate density lipoprotein and VLDL, both of which are associated with an increased risk for atherosclerosis. Given the pivotal role of lipids in the development of plaques, these reports provide ample cause for concern that atherosclerosis may be accelerated or induced by antiretroviral-associated dyslipidemia.
Diabetes mellitus is a major risk factor for the development of premature CAD. In the recently revised Adult Treatment Panel III guidelines from the National Cholesterol Education Project (NCEP ATP III), diabetes mellitus is considered equivalent to having CAD. Insulin resistance in the absence of frank diabetes mellitus has also been associated with the development of CAD. Insulin resistance is associated with a characteristic atherogenic dyslipidemia consisting of elevated triglyceride levels and low HDL cholesterol. Insulin resistance has also been linked with the development of hypertension and the presence of small, dense atherogenic LDL particles. Insulin resistance has been described in the setting of fat-redistribution disorders in HIV-infected patients treated with PIs and NRTIs. Peripheral insulin resistance and impaired oral glucose tolerance occur more frequently in PI-treated individuals compared with treatment-naive individuals and HIV-negative controls in the absence of clinical evidence of fat redistribution.
Endothelial dysfunction correlates with the subsequent development of CAD and may be one of the early changes observed in the development of arterial plaques. Stein demonstrated decreased brachial artery reactivity (endothelial dysfunction) associated with atherogenic dyslipidemia in PI-treated individuals. Similarly, Dube and colleagues demonstrated that short-term administration of indinavir induces endothelial dysfunction in HIV-negative volunteers. Animal models and in vitro data also suggest that NRTIs may diminish endothelial reactivity.
Several surrogate markers have been correlated with the development of symptomatic CAD in HIV-infected individuals. Henry and colleagues demonstrated increased C-reactive protein levels in persons taking indinavir-based therapy. Hadigan and colleagues observed that, compared with controls from the Framingham Heart Study, HIV-infected individuals with fat redistribution had elevated PAI-1 (46.1 vs. 18.9 mug/L) and tPA (16.6 vs. 8.0 mug/L) antigen levels.
Carotid intima thickness (IMT) is a proxy measure for progression of atherosclerosis in the general population. Currier and colleagues conducted a prospective matched case-control study of 135 HIV-infected subjects at low risk for CAD. Each PI-treated patient was matched with a PI-naive HIV-infected individual and an HIV-negative control, according to the following cardiovascular risk factors: age within 5 years; race; gender; blood pressure status (either normal or hypertensive); smoking status (never, current, or former); and menopausal status. Subjects were excluded if they had a family history of MI, diabetes mellitus, prior CAD, or uncontrolled hypertension. There were no differences in the median carotid IMT levels at baseline among the groups (PI group, 0.693 mm; PI-naive group, 0.708 mm; and HIV-negative group, 0.687 mm). Factors associated with increased IMT in multivariate analysis included low HDL cholesterol, elevated triglyceride levels (when HDL was low), older age, and increased BMI. In contrast, Hsue and colleagues found higher baseline mean carotid IMT values (0.9 mm) associated with PI use and that progression of thickness was associated with age and PI use.
Several groups have measured quantitative calcium scores (CAC) in coronary arteries by electron beam computed tomography. Meng and colleagues reported significantly higher CAC scores in PI-treated compared with PI-naive patients (11.0 vs. 1.7; P=0.43). In contrast, Talwani and Acevedo each reported no difference in CAC scores in HIV-infected compared with HIV-negative individuals.
How do we resolve the disparate findings of these studies? The exclusion of subjects with diabetes or a family history of CAD and the closely matched groups studied may explain why Currier's results differ from other studies that have suggested that PI use is associated with the development of atherosclerosis. Of note, the baseline findings in Currier's study do not preclude an effect of therapy-related lipid changes on carotid IMT over longer periods of time. Follow-up was short in all of these studies, precluding any firm conclusions.
MANAGEMENT
In the absence of definitive evidence demonstrating that antiretroviral-associated dyslipidemia, insulin resistance, and endothelial dysfunction do not increase the risk for developing symptomatic CAD, all antiretroviral-treated HIV-infected individuals should be periodically evaluated for relevant risk factors. Evaluation should include assessment of fasting glucose and lipid levels before and after the initiation of antiretroviral therapy, monitoring of blood pressure, and assessment of family history of premature atherosclerosis, history of cigarette smoking, and diet and physical activity. Clinicians should periodically inquire about chest pain or anginal equivalents in HIV-infected patients.
Quantification of CAD risk in HIV-infected individuals has been validated by the Framingham Heart Study. This scoring system is based on blood pressure, fasting lipid levels, age, gender, and cigarette smoking status. Simple risk assessment software is available online from the National Heart, Lung and Blood Institute
( www.hin.nhlbi.nih.gov/atpIII/calculator.asp). Once risk is assessed, individualized interventions can be developed based on general recommendations for CAD risk reduction in HIV-negative adults.
Patients should be encouraged to stop smoking, eat a balanced low-fat, low-carbohydrate diet, and exercise regularly. Hypertension should be treated using the new guidelines issued by the 7th Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure
( www.nhlbi.nih.gov/guidelines/hypertension/index.htm). Guidelines on the specifics of dietary intervention are also available from the NCEP ATP at the same website. Patients over age 40 should consider taking aspirin daily to reduce the risk of a hypercoagulable state that contributes to the development of acute coronary occlusions.
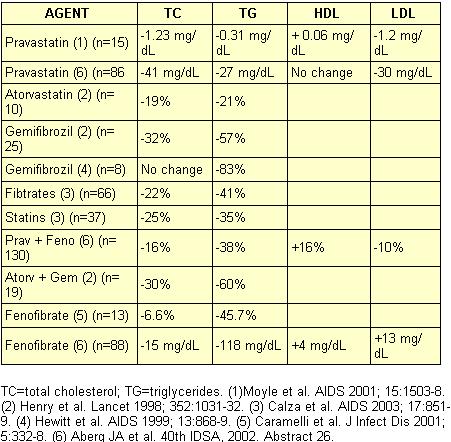
Dyslipidemia remains the most important treatable risk factor for CAD in HIV-infected individuals. The International AIDS Society-USA has issued guidelines for the treatment of metabolic complications, as has the AIDS Clinical Trials Group. These references provide significant detail about the management of these problems. The important principles for treating dyslipidemia include assessment of risk, obtaining fasting lipid levels, targeting interventions based upon the lipid profile, and monitoring response. The primary focus of the NCEP ATP III is the treatment of elevated LDL cholesterol. Most authorities recommend treatment of HIV-infected persons based on data derived largely from the general population. Triglyceride elevations are a secondary target in the new ATP III guidelines, with the focus on reduction of non-HDL cholesterol (total cholesterol minus HDL cholesterol). A simple rule of thumb when using non-HDL cholesterol as a guide to therapeutic intervention is to add 30 mg/dL to the LDL treatment targets. Measurement of non-HDL cholesterol is useful because LDL levels can be unreliable in the setting of triglyceride levels >400 mg/dL. The most common pharmacologic intervention is to use a statin or fibrate (See Table 2). On average, statins reduce cholesterol from 20% to 40% and triglyceride levels to a lesser degree, whereas fibrates reduce triglycerides to a greater extent (40% to 50%) and cholesterol by around 10% to 15%. Thurs, fibrates are generally recommended initially for the treatment of hypertriglyceridemia, whereas statins are initially used for hypercholesterolemia. In the absence of clinical endpoint data, it seems prudent to treat significant dyslipidemia in HIV-infected individuals. Clinicians should remember there are important drug interactions between both statins and fibrates on the one hand and specific antiretroviral drugs on the other hand. For example, simvastatin and lovastatin plasma levels are extremely elevated with concomitant use of PIs, and these agents are therefore contraindicated by PI use.
|
|
| |
| |
 |
|
| |
| |
FUTURE RESEARCH
The focus of research on atherosclerosis in HIV-infected individuals remains on determining the presence and nature of any association with the use of antiretroviral therapy. The prevalence of known CAD risk factors in the HIV-infected population may obscure the role of antiretroviral therapy. Longitudinal studies with matched controls are needed to measure the rate of atherosclerosis development while controlling for other known risk factors. Such studies are obviously complicated by the frequent need to adjust antiretroviral regimens. It may not be possible to design a human study that can truly answer the cause-and-effect question. However, much can be learned from animal models of atherosclerosis. These studies are underway and should provide further insight on the effects of antiretroviral therapy on the development of arterial plaques.
CONCLUSIONS
The jury is still out as to whether antiretroviral therapy induces or accelerates the development of atherosclerosis. Nevertheless, the preponderance of the current evidence suggests that there is reason to be concerned and that interventions to reduce risk are warranted. Clinicians should screen aggressively for CAD risk factors. Emphasis should be placed on risk reduction, and treatment of CAD should be initiated when appropriate using standard guidelines developed for the general population.
Dr. Fichtenbaum is Associate Professor of Clinical Medicine University of Cincinnati College of Medicine.
FURTHER READING
Aberg JA et al. A prospective, multicenter, randomized trial comparing the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: ACTG 5087. 40th Annual Meeting of Infectious Diseases Society of America, Chicago, October, 2002. Abstract 26.
Bozzette SA et al. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med 2003 Feb 20; 348:702-10.
Carr A et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998 May 7; 12:F51-8.
Carr A et al. Diagnosis, prediction, and natural course of HIV-1protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 1999 Jun 19; 353:2093-9.
David MH et al. Ischemic cardiovascular disease in persons with human immunodeficiency virus infection. Clin Infect Dis 2002 Jan 1; 34:98-102.
Dube MP et al. Preliminary guidelines for the evaluation and management of dyslipidemia in adults infected with human immunodeficiency virus and receiving antiretroviral therapy: Recommendations of the Adult AIDS Clinical Trial Group Cardiovascular Disease Focus Group. Clin Infect Dis 2000 Nov; 31:1216-24.
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001 May 16; 285:2486-97.
Fichtenbaum CJ et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS 2002 Mar 8; 16:569-77.
Flynn TE and Bricker LA. Myocardial infarction in HIV-infected men receiving protease inhibitors. Ann Intern Med 1999 Oct 5; 131:548.
Friis-Moller N et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS 2003 May 23; 17:1179-93.
Grundy MS et al. Primary prevention of coronary heart disease: Guidance from Framingham: A statement for healthcare professionals from the AHA Task Force on Risk Reduction. Circulation 1998 May 12; 97:1876-7.
Hadigan C et al. Increased PAI-1 and tPA antigen levels are reduced with metformin therapy in HIV-infected patients with fat redistribution and insulin resistance. J Clin Endocrinol Metab 2001 Feb; 86:939-43.
Haffner SM. Epidemiology of insulin resistance and its relation to coronary artery disease. Am J Cardiol 1999 Jul 8; 84:11J-4J.
Henry K et al. Atorvastatin and gemfibrozil for protease-inhibitor-related lipid abnormalities. Lancet 1998 Sep 26; 352:1031-2.
Henry K et al. Severe premature coronary artery disease with protease inhibitors. Lancet 1998 May 2; 351:1328.
Klein D et al. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr 2002 Aug 15; 30:471-7.
Lamarche B. Abdominal obesity and its metabolic complications: implications for the risk of ischaemic heart disease. Coron Artery Dis 1998; 9:473-81.
Miller KD et al. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet 1998 Mar 21; 351:871-5.
Passalaris JD et al. Coronary artery disease and human immunodeficiency virus infection. Clin Infect Dis 2000 Sep; 31:787-97.
Riddler SA et al. Impact of HIV infection and HAART on serum lipids in men. JAMA 2003 Jun 11; 289:2978-82.
Schambelan M et al. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: Recommendations of an International AIDS Society-USA panel. J Acquir Immune Defic Syndr 2002 Nov 1; 31:257-75.
Segerer S et al. Hyperlipidemia under treatment with protease inhibitors. Infection 1999 Mar-Apr; 27:77-81.
Sullivan AK et al. Coronary artery disease occurring with protease inhibitor therapy. Int J STD AIDS 1998 Nov; 9:711-2.
|
|
| |
| |
|
|
|