| |
|
Boehringer Ingelheim Launches Global Phase III Clinical Trials For New Anti-HIV Agent Tipranavir
|
| |
| |
Clinical Trial Program is Largest Ever for HIV-Positive Patients Previously
Treated with Three Classes of Antiretrovirals
RIDGEFIELD, Conn., Feb. 6 /PRNewswire/ -- Boehringer Ingelheim today announced the launch of the Phase III RESIST clinical trial program designed to further study the efficacy and safety of tipranavir for use in combination therapy for HIV-1 infection. The RESIST 1 & 2 trials and accompanying companion studies will evaluate triple class-experienced patients(1) in more than 280 clinical trial sites worldwide. Tipranavir is the first non-peptidic protease inhibitor (NPPI) in development for the treatment of HIV-1 infection. It will be Boehringer Ingelheim's second antiretroviral drug following Viramune(R) (nevirapine), which is a non-nucleoside reverse transcriptase inhibitor.
Resistance to currently available anti-HIV drugs is an increasingly prevalent concern for highly treatment-experienced HIV-positive patients worldwide, creating an urgent need for new treatments that are effective against multi-drug resistant virus. Up to 14% of recently infected patients have been reported to be infected with a strain of virus that has drug resistance mutations or that has reduced susceptibility (effectiveness) to a particular drug(2), (3), (4). Available clinical and in vitro data suggest that tipranavir may be active against strains of HIV-1 that are resistant to currently available peptidic protease inhibitors (PIs), offering hope for patients with multi-drug resistant virus. Phase III trials are designed to confirm these initial studies.
"This is an important milestone in the development of tipranavir," said Dr. Andreas Barner, Member of the Board of Managing Directors of Boehringer Ingelheim. "We have designed a clinically relevant Phase III trial program that includes resistance testing for all patients, allows the use of other expanded access HIV/AIDS therapies, and provides access to a significant number of triple class-experienced patients worldwide. It's the largest trial program ever conducted in this patient population, recruiting over 1500 patients in total."
The Phase III RESIST (Randomized Evaluation of Strategic Intervention in Multi-Drug ReSistant Patients with Tipranavir) clinical trial program has been designed to study the safety and efficacy of tipranavir (boosted with low-dose ritonavir) versus a low-dose ritonavir-boosted comparator protease inhibitor (PI) that is chosen by the patient's physician on the basis of treatment history and baseline resistance testing. Study participants will all be highly treatment-experienced HIV-positive adults. Phase III of clinical development is the final stage of testing before a drug is submitted to worldwide regulatory authorities for review and consideration for marketing approval. The RESIST Program consists of two Phase III pivotal trials (RESIST 1 and RESIST 2) and two companion trials (study 1182.51 and RESIST 3) available for even more advanced patients.
"The impressive activity seen in initial studies demonstrate that tipranavir may be a promising option for patients with existing PI-resistant virus. I look forward to seeing the outcome of these important Phase III trials," said Kathleen Squires, MD, Associate Professor of Medicine at the Keck School of Medicine of the University of Southern California, and an investigator in the RESIST program.
In initial clinical studies, the most common adverse events associated with tipranavir were diarrhea, nausea, fatigue, headache and vomiting. In addition, the elevation of transaminases has been observed in patients treated with tipranavir.
RESIST Clinical Trial Program Design
RESIST 1 will enroll more than 500 patients at more than 115 trial sites in the United States, Canada and Australia. All participants will receive resistance testing at baseline to determine the optimal drugs to combine with tipranavir, as each patient will receive an individualized background treatment regimen. The choice of comparator PI and background regimen is up to the investigator. A similar clinical trial, RESIST 2, will enroll more than 800 patients in Europe and South America.
HIV/AIDS healthcare providers interested in finding a local RESIST trial site can access a list of U.S. sites at http://us.boehringer-ingelheim.com or http://www.clinicaltrials.gov.
Companion studies for patients with extremely limited treatment options will also be available at participating RESIST study sites for patients who do not qualify for the RESIST trials. Boehringer Ingelheim will also study tipranavir in treatment-naive adult patients and conduct trials in children. The dose for tipranavir that will be studied in the Phase III clinical program is 500 mg of tipranavir taken with 200 mg of ritonavir (to boost tipranavir drug levels in the body) twice daily.
Tipranavir
Tipranavir has a non-peptidic chemical structure, which is believed to allow it to bind more flexibly to the active site of the HIV protease. This flexibility may explain why the resistance profile of tipranavir is different from available peptidic PIs. While the exact resistance profile of tipranavir is still being defined, initial clinical and in vitro data suggest that tipranavir may be active against strains of HIV-1 that are resistant to currently available peptidic protease inhibitors. Preliminary results from Phase II clinical studies, which require confirmation by the RESIST trial program, suggest that reduction in the susceptibility of HIV-1 to tipranavir is uncommon and is associated with at least 16 protease inhibitor mutations at baseline.
HIV Drug Resistance
The HIV-1 virus acquires HIV drug resistance after sub-optimal exposure to antiretroviral therapy, which becomes more of an issue the longer patients are receiving treatment. Drug resistance may render drugs less effective, or even ineffective, thus significantly limiting treatment options for people with HIV/AIDS. Resistance generally occurs as a result of changes -- or mutations -- in HIV's genetic code.
Boehringer Ingelheim
Boehringer Ingelheim is committed to the research and development of novel antiretroviral agents. VIRAMUNE (nevirapine) is a product of original research done at Boehringer Ingelheim. VIRAMUNE was the first member of the non-nucleoside reverse transcriptase inhibitor (NNRTI) class of anti-HIV drugs. Boehringer Ingelheim is committed to the rapid development of the investigational non-peptidic protease inhibitor tipranavir. The company is involved in basic research and is committed to improving HIV therapy by providing physicians and patients with innovative antiretrovirals.
For more information on Boehringer Ingelheim, please see www.boehringer- ingelheim.com/hiv.
References:
(1) Previously treated with three classes of antiretrovirals: nucleoside
analogues, non-nucleoside reverse transcriptase inhibitors and
protease inhibitors.
(2) Boden D, Hurley A, Zhang L, Cao Y, Guo Y, Jones E, et al. HIV-1 drug
resistance in newly infected individuals. JAMA 1999;282: 1135-41.
(3) Little SJ, Daar ES, D'Aquila RRT, Keiser PII, Connick E, Whitcomb JM,
et al. Reduced antiretroviral drug susceptibility among patients with
primary HIV infection. JAMA 1999; 282:1142-9.
(4) Yerly S, Kaiser L, Race E, Bru JP, Clavel F, Perrin L. Transmission
of antiretroviral-drug-resistant HIV-1 variants. Lancet 1999; 354: 729-33.
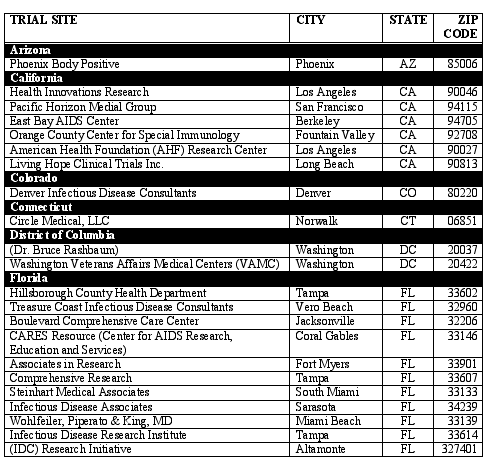
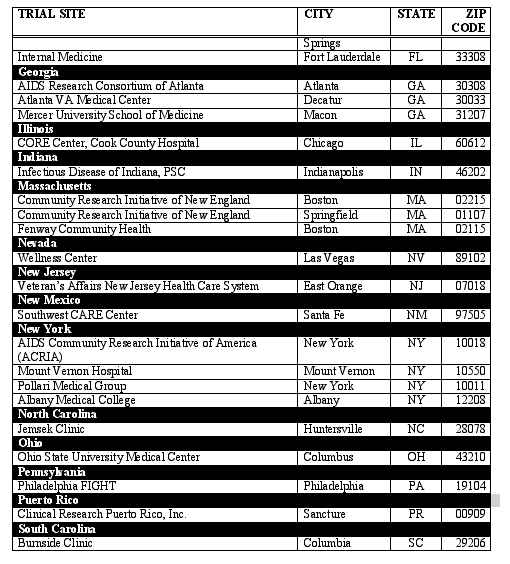
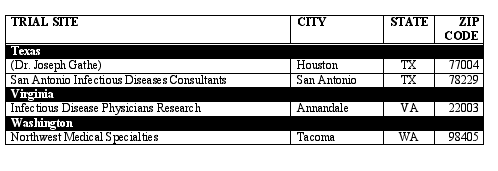
Fully-Contracted U.S. RESIST Trial Sites
Last Updated on 2/6/03
The RESIST clinical trial program will enroll patients at more than 115 sites in the U.S. Following is a list showing only fully-contracted trial sites as of February 6, 2003. This list will be updated periodically as contracts with additional sites are finalized.
If you would like information on how to enroll in RESIST, please locate in the below list the trial site nearest to you and contact the site directly.
|
|
 |
| |
 |
| |
 |
| |
|
|
|