| |
72 weeks HCV Therapy (PegIFN/RBV): is it effective?
|
| |
| |
"Extending combination therapy with peginterferon alfa-2b plus ribavirin for genotype 1 chronic hepatitis C late responders: A report of 9 cases"
In a letter to the editor in the May issue of the journal Hepatology (May 203, Volume 37, Number 5), researchers from Spain and Israel treated 9 selected patients for 72 weeks and 7/8 achieved a sustained viral response (SVR). These patients achieved undetectable HCV RNA between 12 and 24 weeks on therapy. The authors concluded that further research should be conducted to see if longer duration therapy can improve response rates. The study is small but important. Findings from a previously conducted study (The Benelux Study) which was presented at the European hepatitis conference (EASL) suggested similarly that longer duration therapy of 18 months may improve response rates, particularly in hard to treat patient populations; such as previous non-responders and partial responders, people with HIV, and genotype 1. I believe a study is ongoing now to explore this question but further research is needed. This is an important concept that may improve response rates in difficult to treat cases. Personally, I was a previous non-responder to IFN/RBV, have HIV and had cirrhosis. I completed 18 months PegIFN/RBV in August 2002. Six months after stopping therapy my viral load was undetectable. Of course there is no way to determine if I would have achieved this success with 12 months therapy as well. But extending therapy may be crucial for some individuals. So further research needs to be conducted so medical care providers can have data upon which to base this decision.
To the editor:
The large number of chronic hepatitis C patients infected by genotype 1 constitutes an important therapeutic challenge because they are the
most resistant to combination therapy with interferon alfa and ribavirin. In these patients, the sustained virologic response to peginterferon and ribavirin for 48 weeks is around 42%. It has been shown that one important factor of sustained virologic response (SVR) is rapid hepatitis C virus (HCV)-RNA clearance, ranging from 75% for those patients who cleared the virus at week 12 to only 32% for those who lost HCV RNA at week 24. It has been suggested that extending therapy in patients who cleared HCV RNA between weeks 12 and 24 (i.e., late responders) could increase SVR.
We selected 9 patients with chronic hepatitis C who were infected with genotype 1 in treatment with pegylated interferon alfa-2b plus ribavirin
who cleared HCV RNA between weeks 12 and 24 for therapy prolonged to 72 weeks. Three were men and 6 were women, with a median age of 41 ± 14.57 years. All patients had elevated alanine aminotransferase levels, positive HCV RNA, and a liver examination showing chronic hepatitis. Patients were treated with a mean dose of 1.0 µg/kg of peginterferon alfa-2b once weekly (PEG-INTRON, Schering-Plough, Kenilworth, NJ), plus 800 mg/d of ribavirin (Rebetol, Schering-Plough). HCV RNA was analyzed by using a quantitative, real-time, reverse-transcriptase polymerase chain reaction technique with a lower limit of detection of 100 IU/L.4
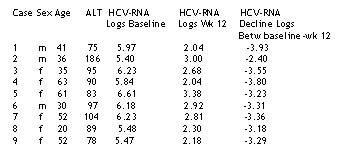
Eight patients completed therapy and 6 months of follow-up. One patient stopped therapy at week 48 because of thyroid alterations. Table 1 shows patient characteristics and changes in HCV-RNA levels from baseline to week 12.
Table 1. Baseline characteristics of the 9 patients treated and HCV-RNA changes in logs from baseline to week 12 and HCV-RNA decline from baseline to week 12. All 9 patients had chronic hepatitis C, and 8 of the 9 had moderate stage histology. Patient 3 had mild chronic HCV stage disease. HCV-RNA logs are in IU/ml unit of measure. To give you some understanding of the log values: 5.97 HCV-RNA logs is about 1 million IU/ml; 6.61 HCV-RNA logs is about 4 million; 5.4 logs is about 250,000 IU/ml; 6.18 is 1.5 million. Under 2 million is consider a low viral load. In large studies patients with low viral load tend to respond better to therapy, but in this small study patients with high viral load responded as well as patients with lower viral load. Perhaps the extended duration of therapy helped.
|
|
 |
| |
RESULTS: In all patients, HCV RNA was positive at week 12 of therapy but undetectable at week 24 and throughout the 72 weeks of therapy. At week 24 of follow-up, 7 patients maintained an SVR and one relapsed (case 3).
This study, with a small number of patients, showed that prolonged combination therapy with peginterferon and ribavirin is very useful in late
virologic responders because it increases SVR. HCV-RNA determination has an important role not only in the decision to stop therapy but also in
better adjusting therapy. Currently, nonresponders can be detected by a quantitative HCV-RNA test at week 12, showing a decline of less
than 2 logs in HCV-RNA concentrations. In these patients, combination therapy should be stopped because the probabilities of a sustained
response are almost nil. In patients who achieve an early virologic response, the probabilities of achieving an SVR were 80% for those who cleared HCV RNA at week 12 and sooner, and 40% for those who achieved a 2-log reduction in HCV-RNA concentrations but still remained HCV-RNA positive as a recent review of multicenter studies has shown.5 All of our patients had a 2-log decline but remained HCV-RNA positive at week 12 and, taking into account the previous results, their probabilities of achieving an SVR are lower than those patients who were HCV-RNA negative at week 12. In this subgroup of patients, with a slower decline in HCV-RNA levels, usually genotype 1 patients with high
baseline viral levels, continuing therapy to 72 weeks could be the best way to ensure an SVR with acceptable tolerability and safety.
In summary, extending therapy with peginterferon alfa plus ribavirin to 72 weeks for late virologic responders may induce a higher SVR. These
results merit further prospective, randomized, controlled studies, using the optimal doses of peginterferon and ribavirin for longer duration versus
the current standard 48-week therapy in this subset of patients.
Maria Buti, M.D.
Auristela Valdes
Francisco Sanchez-Avila
Rafael Esteban, M.D.
Liver Unit, Hospital General Universitari Vall d'Hebron, Barcelona, Spain
Yoav Lurie, M.D.
Liver Clinic, Gastroenterology Institute, Kaplan, Israel
|
|
| |
| |
|
|
|