| |
Treating hepatitis C in methadone maintenance patients: an interim analysis
|
| |
| |
This article was published in July 2002 in the journal called Drug and Alcohol Dependence by Diana Sylvestre, MD. It was written and submitted to the journal prior to more recent study results. Recently updated results available are on my website in report on public presentation by Sylvestre at DDW Conference 2002; here is link to article:
HCV Therapy in Methadone Maintenance
http://www.natap.org/2002/DDWLiver/day1.htm
Updated Study Results from Jules Levin
(following these 4 slides is the text of the published article which provides interesting background information.)
|
| |
| |
 |
|
| |
| |
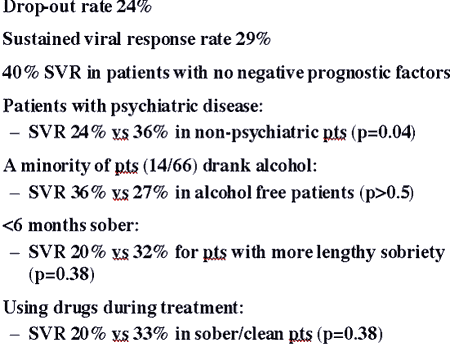 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
PULISHED ARTICLE
Drug and Alcohol Dependence Volume 67, Issue 2, 1 July 2002, Pages 117-123
Diana L. Sylvestre, Department of Medicine, University of California, San Francisco, Organization to Achieve Solutions in Substance-Abuse (O.A.S.I.S.), 2862 Telegraph Avenue, Oakland, CA 94609, USA
Abstract
Objective: This study evaluates the safety, tolerability, and efficacy of interferon/ribavirin combination therapy in methadone maintenance (MM) patients with active hepatitis C (HCV). End-of-treatment results are presented as an interim analysis of efficacy.
Methods: Fifty eligible MM patients with active HCV and concomitant liver fibrosis were treated with interferon/ribavirin combination therapy using standard dosing regimens. Patients with active drug or alcohol use at the start of treatment, severe or untreated psychiatric illness, and/or decompensated liver disease were excluded.
Results: Treated MM patients were older, had a longer history of HCV infection, a high prevalence of psychiatric illness, and had substantially more liver fibrosis than in previous studies of HCV treatment in non-opioid-dependent populations. Seventy-eight percent completed the 6–12 month course of treatment. The end-of-treatment virologic response rate was 64% in patients completing treatment and 54% on an intent-to-treat basis. Conclusion: Methadone maintenance patients exhibit a number of factors that make HCV treatment more difficult: they are older, have a higher prevalence of psychiatric illness, and show evidence of more advanced liver disease. Despite this, their end-of-treatment response rate to interferon/ribavirin combination therapy is similar to that of patients without a history of IDU. Further analysis of the sustained virologic response (SVR) rate is planned at the study's completion. These preliminary results show that MM patients are in need of timely HCV diagnosis, and should be considered good candidates for referral and HCV treatment.
1. Introduction
Hepatitis C (HCV) is the most common blood borne infection in the United States (Centers for Disease Control and Prevention, 1998); approximately 4 million people in the US and over 170 million worldwide have been exposed. Of these, 75–85% will develop a chronic, active infection ( Alter et al., 1999). HCV is the leading cause of chronic liver disease and the most common indication for liver transplantation in adults ( Detre et al., 1997).
Injection drug use (IDU) currently accounts for the majority of new cases of HCV, and approximately 70–96% of IDUs are infected with the virus (Esteban; Donahue; Zeldis; Garfein; Hershow and McCarthy). Parenteral transmission is very efficient: as many as 65% of IDUs are infected within 1 year of needle use, and after 5 years of injecting as many as 90% of users are infected with HCV ( Garfein et al., 1996). The highest prevalence of HCV infection is found among those with large or repeated percutaneous exposures to blood, and transmission has been linked not just to needle sharing, but also to the sharing of other parts of the injection paraphernalia ( Hagan et al., 2001).
Although the progression of HCV liver disease is quite variable, it typically advances slowly over a period of decades. According to most studies, about 20% of patients with chronic HCV infection will develop cirrhosis within 20–30 years. Once cirrhosis develops, however, the risk of decompensation is about 3–4% per year and the risk of liver cancer is about 1.5% per year (Di Bisceglie et al., 1991).
Many of those infected with HCV are minimally symptomatic or asymptomatic until the liver disease is advanced. Common symptoms are non-specific and include fatigue, muscle and joint aches, nausea, abdominal pain and headaches. Only about half of those infected will have persistently elevated liver function enzymes, and even patients exhibiting normal blood tests can have histologic evidence of inflammation and fibrosis (Jamal et al., 1999). The diagnosis must therefore be based on a high suspicion for the disease based on risk factor assessment.
The treatment of HCV has evolved rapidly. The first agent approved for treatment was interferon , which as monotherapy induced a sustained biochemical and virologic response in less than 20% of treated patients (Poynard et al., 1996). More recently, the addition of oral ribavirin has led to a substantial improvement in virologic response rates. A typical treatment regimen now consists of 6–12 months of interferon/ribavirin combination therapy. Approximately 40% of patients who complete the treatment will exhibit a sustained virologic response (SVR), as demonstrated by the absence of virus 6 months after the completion of therapy ( Poynard and McHutchison). Importantly, over 95% of these patients will remain virus-free 5 years later and may be cured of the disease ( Reichard et al., 1999). In addition, the US FDA has recently approved a longer acting form of interferon, called pegylated interferon, which elicits a SVR in nearly 40% as a monotherapy (Zeuzem et al., 2000). Studies combining this interferon with ribavirin have resulted in overall sustained response rates of 54–56% ( Fried and Manns).
Unfortunately, interferon/ribavirin combination therapy is expensive and can be physically arduous. Interferon often causes flu-like symptoms and cytopenias. It may lead to a depression that may be so severe as to result in suicide (Musselman et al., 2001). Ribavirin can cause a severe hemolytic anemia, and many patients exhibit a host of more minor side effects. Despite these drawbacks, however, combination therapy is the best option available for patients who demonstrate progressive HCV-related liver disease. Patients with advanced liver disease may not be interferon/ribavirin treatment candidates because the medications may cause hepatic decompensation, and at that point liver transplantation is the only option. Since the availability of liver transplants is limited, initiating treatment in a timely fashion is critical.
Despite the remarkably high prevalence of HCV in IDU, there are surprisingly little data about treatment in this population. Interferon/ribavirin treatment is expensive and time-consuming, and IDUs as a group tend to be relatively underinsured and have limited access to quality health care services. Even those in stable recovery are often considered poor treatment candidates due to a variety of factors including pre-existing psychiatric illness and comorbid medical conditions. In addition, many physicians are unwilling to prescribe a medical treatment to IDUs that includes injections. For these reasons, methadone patients have been excluded from the majority of HCV treatment studies, leading to an incomplete understanding of the use of interferon and ribavirin in recovering IDUs with progressive HCV.
Organization to Achieve Solutions in Substance-Abuse (O.A.S.I.S.) is a non-profit organization located in Oakland, CA that has developed a unique group HCV treatment program for recovering IDUs. As a referral base for the greater San Francisco Bay Area, it draws from over 4500 methadone maintenance patients, and approximately 900 of these have been evaluated for disease activity and progression. A focus of O.A.S.I.S. is to understand the impact of current and former IDU on the natural history of hepatitis C and its treatment. These results are an interim analysis of the first 50 patients to complete interferon/ribavirin combination therapy in our pilot study of HCV treatment in MM patients with active HCV and hepatic fibrosis. This report describes their demographic and clinical characteristics, treatment adherence, and end-of-treatment virologic response rates, comparing these to large published studies of HCV treatment in non-opioid-dependent populations.
2. Methods
2.1. Study design
This study is part of an ongoing 72-week open-label study of standard interferon/ribavirin combination therapy in methadone maintenance patients with active HCV and liver fibrosis. Treatment consists of interferon -2b, 3 x106 U administered subcutaneously three times weekly and ribavirin capsules, 1000 mg taken orally daily in divided doses for patients weighing less than 165 pounds, or 1200 mg daily for those weighing 165 pounds or more. Patients with genotype 1 receive 48 weeks of therapy, and genotype non-1 receive 24 weeks of therapy if in virologic remission at that time point, or 48 weeks if they exhibit detectable virus at the 24-week milestone. All medications were self-administered, and adherence to therapy was assessed monthly by a questionnaire that detailed the number of doses of medication that had been missed in the prior month.
The primary endpoint of this analysis is the end-of-treatment virologic response, indicated by undetectable levels of HCV RNA on analysis with the Bayer HCV RNA bDNA 3.0 assay with a lower limit of detection of 550 IU/ml. Patients are classified as responders if they have no detectable virus at the completion of therapy, as non-responders if they exhibit detectable virus at all measured intervals, or breakthrough non-responders if they have no detectable virus at some point during therapy but are virus positive at the completion of therapy.
Secondary outcomes include medication safety and side effect profiles, treatment retention rates, and the impact of treatment on methadone dosing and illicit drug use. The protocol was evaluated and approved by the Ethical Review Committee, Kansas City, MO.
2.2. Inclusion criteria
Males and females ages 18 and older are eligible if they have been on MM treatment and have a positive HCV antibody test. Those with active HCV as documented by a positive PCR are treatment candidates if they demonstrate liver fibrosis either by biopsy or by surrogate blood markers of fibrosis: thrombocytopenia/25% reduction in platelet count compared with a historical baseline, hypoalbuminemia/25% reduction in albumin, and/or splenomegaly.
2.3. Exclusion criteria
Patients with evidence of active decompensated liver disease, i.e. current ascites, variceal bleeding within 3 months, platelet counts of <50000, an albumin of <3.0, and spontaneous encephalopathy are excluded. Uncontrolled moderate-severe depression as determined by a Center for Epidemiologic Studies Depression Scale (CES-D) score of >24 is an exclusion until the disease is stabilized with medications (Weissman et al., 1977). Patients with active drug and/or alcohol abuse at enrollment are excluded; the use of marijuana under medical supervision (`medical marijuana') was not an exclusion. Patients with non-HCV-related liver disease such as antigen-positive hepatitis B or autoimmune disease are excluded, as are patients of childbearing potential unwilling or unable to use two reliable forms of birth control. Other exclusion criteria include severe cardiac, pulmonary, renal, or retinal conditions.
2.4. Procedures
Informed consent was obtained, and participants completed a self-administered questionnaire delineating baseline demographic, psychosocial, psychiatric, and substance use characteristics. An estimate of the length of HCV infection was made on the basis of the number of years of IDU minus one, and the years of heavy alcohol use, defined as a daily consumption of two alcoholic beverages (20 g) or more, were based on patient report. Other demographic characteristics were based on patient report.
Baseline assessments including liver function tests, complete blood counts (CBC), TSH, HCV PCR, HCV genotype, and the CES-D depression scale were performed. Liver biopsy was optional for patients exhibiting surrogate markers of fibrosis: a platelet count of <150000, albumin <3.5, and/or splenomegaly. Patients without surrogate markers of liver fibrosis were referred to a local medical center where ultrasound-guided liver biopsy was performed by interventional radiologists under local anesthesia. Biopsy specimens were examined by a hospital pathologist unfamiliar with the patient and fibrosis was scored on a scale of 0–4, with 0, none; 1, minimal-mild; 2, mild-moderate; 3, moderate-severe; and 4, cirrhosis.
After treatment initiation, patients were monitored with CBC, electrolyte panel, and liver functions at a minimum of 2, 4 and every 4 weeks thereafter throughout the course of the treatment. Thyroid stimulating hormone (TSH) was tested every 12 weeks. Those with side effects were monitored more frequently until their treatment stabilized. Drug and alcohol consumption were monitored by monthly self-report questionnaires, and monthly random urine drug test results were obtained from the subject's methadone treatment program. The CES-D test was performed every 12 weeks and worsening psychiatric disease was treated with anti-depressants; SSRI's were used as first-line agents. HCV RNA PCR was performed every 12 weeks. Active participation in peer support/education groups throughout the treatment course was encouraged.
The interferon dose was adjusted or discontinued for clinically significant thrombocytopenia, leukopenia, and neutropenia and the ribavirin was adjusted on the basis of anemia using standard published protocols. Side effect management included the intake of 15–20 8 oz. glasses of water daily and 50 mg diphenhydramine with 600–800 mg ibuprofen taken 30 min prior to interferon dosing. Diphenhydramine and ibuprofen were also taken in low dose as needed, and low dose acetaminophen was prescribed to ibuprofen-intolerant patients. Patients experiencing significant withdrawal-like symptoms were encouraged to contact their methadone provider to increase their methadone dose minimally to improve their comfort level.
The treatment was discontinued if requested by the patient or for severe cytopenias, uncontrolled and/or worsening psychiatric conditions, or decompensating liver disease.
2.5. Data and statistics
All data were compiled in and analyzed using version 10 for Windows. The mean, median, and S.E.M. were calculated for relevant clinical characteristics; bivariate analysis of categorical data was performed with 2 and Fisher exact tests, with a P value less than 0.05 in two-tail comparison considered statistically significant. Study results obtained from large historical studies of HCV treatment in non-opioid-dependent populations were used as a comparator group (Poynard and McHutchison).
3. Results
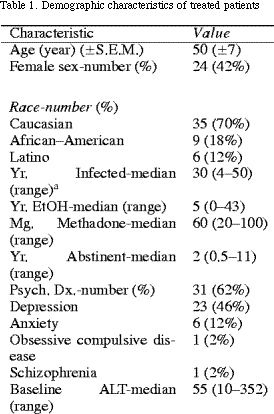
A total of 50 patients have met the criteria for treatment and have completed the course of therapy. Thirty-one of these patients (62%) had surrogate markers of fibrosis and 19 (38%) had a liver biopsy documenting fibrosis. Table 1 gives the demographic characteristics of these patients with regard to age, sex, race/ethnicity, genotype, length of infection, years of heavy alcohol use, and psychiatric conditions. The majority of patients were male, and minorities represented 30% of the treated subjects. Age at the start of treatment ranged from 32 to 66 years, with an average age of 50 years.
|
| |
| |
 |
|
| |
| |
The estimated average length of infection ranged from 4 to 50 years with a median of 30 years, and heavy alcohol consumption ranged from 0 to 43 years with a median of 5 years. As seen in Table 1, of the 31 patients (62%) reporting a history of psychiatric diagnosis, the majority had been diagnosed with depression of anxiety. All conditions were considered stable at the initiation of treatment. The median dose of methadone was 60 mg (range 20–100 mg). Patients reported being drug-free for 6 months–11 years, with a median of 2 years.
Twenty-eight patients (56%) exhibited an abnormal ALT (>50 IU/l) at the time of screening, with a range of 10–352 IU/l. Eleven patients (22%) were diagnosed with cirrhosis or transition-to-cirrhosis on the basis of a baseline platelet count of less than 100000 and 27 (54%) had baseline thrombocytopenia (<150000). Nineteen patients underwent biopsy; the mean inflammation grade and fibrosis stage for these patients were 2.5 and 2.4, respectively.
The genotype was known in 48 of the patients. Of these, 52%, (25) had genotype 1 and 48% (23) had a genotype other than 1. One patient was HIV positive; her disease was stable on HAART therapy.
Twenty-eight patients (56%) were receiving psychiatric medications at the start of treatment; of these, the most commonly prescribed medications were SSRI's (29%) and benzodiazepines (25%). Thirty-one patients (62%) received a new psychiatric medication during treatment; 16 of these (32%) had not been taking psychiatric medications at the start of treatment. SSRI's were the most commonly prescribed medication during treatment, in 21 (68%) of patients. Overall, 44 patients (88%) were taking a psychiatric medication by the end of treatment.
The most commonly reported side effects were fatigue in 78%, depression/irritability in 70%, nausea in 54%, and flu-like symptoms in 38%. Side effects were generally mild and conservatively managed. Overall, 11 subjects (22%) discontinued treatment. Of this group, three (27%) discontinued due to medication side effects, three (27%) due to worsening psychiatric disease, three (27%) due to decompensating liver disease, and one (9%) due to alcohol abuse. One subject relocated to another state and was unable to obtain medication. Eight of 11 subjects (73%) discontinuing treatment had a pre-existing psychiatric diagnosis, a disease rate not significantly different from that in the total sample.
Thirty-nine patients (78%) completed treatment. Table 2 shows end-of-treatment virologic responses for all subjects, for those completing treatment, and as broken down by genotype. Overall, 27 patients (54%) were end-of-treatment responders and 23 (46%) were non-responders, with five of the 23 (10%) categorized as breakthrough non-responders. For those completing treatment, 25 (64%) were responders and 14 (36%) were non-responders. The 54% treatment response rate based on the intent to treat sample is not significantly different from the 51% rate seen in often clinical trials (P>0.5).
|
| |
| |
 |
|
| |
| |
As can be seen, genotype influenced the response rate greatly. Thirty-six percent of patients with genotype 1 had a complete end-of-treatment virologic response as compared with 70% of non-genotype 1 patients. Both patients with unknown genotypes were responders. The single subject coinfected with HCV and HIV had genotype 1 and was a non-responder.
4. Discussion
This preliminary study demonstrates that, although MM patients exhibit a number of negative prognostic factors, they can undergo interferon/ribavirin combination treatment for hepatitis C with safety, tolerability, and efficacy comparable to that of non-opioid dependent patients. The study subjects exhibited a number of factors that might be expected to negatively impact upon treatment outcomes, including advanced age, advanced liver disease, the relatively high prevalence of African–Americans, and a high prevalence of comorbid psychiatric illness. However, this study population is fairly representative of a large proportion of HCV-infected persons in the US, 60% of whom are current or ex-drug users.
The average age of the subjects, 50 years, is nearly 10 years older than subjects in the pivotal hepatitis C registration trials and they had been infected nearly a decade longer (Poynard and McHutchison). Both of these factors have been shown to reduce HCV treatment response rates. In addition, patients with advanced liver disease can be difficult to treat due to hepatic decompensation, and our study subjects exhibited significantly more liver fibrosis, as demonstrated by surrogate as well as histological markers, than the pivotal treatment studies where the percent of patients with compensated cirrhosis was typically 4% or less. Twenty-two percent of our patients began treatment with a platelet count of <100000, indicative of cirrhosis or transition-to-cirrhosis, and 54% of patients overall had baseline thrombocytopenia, indicative of advanced fibrosis and portal hypertension. Of those who underwent biopsy in our study, the average fibrosis stage was 2.5, significantly higher than the pivotal treatment trials where the fibrosis stage on average was less than 1.5 ( Poynard et al., 1998).
The advanced liver disease exhibited by our patients is not entirely surprising since the greater length of infection and the extensive history of heavy alcohol consumption are both contributing predictors. Other factors to consider are the potential influence of hepatotoxins coinjected at the time of drug use and the impact of the impaired immune status that has been reported in heroin addicts (Novick et al., 1989). Although these observations are interesting, liver biopsy is less commonly performed as HCV medications become more effective. Therefore, in light of the expense of liver biopsy and its potential risk of bleeding or death, HCV treatment may be undertaken without a pretreatment liver biopsy in many patients in whom the results will not influence treatment.
Subjects in this study were also more ethnically diverse than the pivotal treatment studies, with 12% of treated patients being Latino and 18% being African–American. This compares with 5% or less in the majority of published treatment studies. African–Americans have a lower response rate to treatment than other races, in large part although not entirely due to a higher prevalence of genotype 1 (Reddy et al., 1999).
Not all the patient characteristics, however, would be expected to negatively predict treatment outcome. In this study, the prevalence of genotype 1 was 52%, modestly lower than interferon/ribavirin registration trials, in which 57–72% of subjects exhibited genotype 1. As genotype 1 is substantially less responsive to interferon/ribavirin combination therapy, the lower prevalence of this genotype in our study likely enhanced our end-of-treatment response rate, thereby counterbalancing the negative prognostic factors mentioned above. Indeed, the large difference in response rates among the genotypes and the impact of genotype on treatment length reaffirms the importance of obtaining this test despite its expense.
The 22% treatment discontinuation rate approximates that seen in the pivotal HCV treatment trials, despite the remarkably high prevalence of pre-existing psychiatric disease and more advanced liver disease exhibited by the subjects in this study. Treatment side effects were also largely consistent with previously published studies. Aggressive side-effect management including extra water intake, ibuprofen or acetaminophen, diphenhydramine, and methadone dose adjustments likely enhanced the tolerability of this relatively difficult medication regimen and improved treatment adherence.
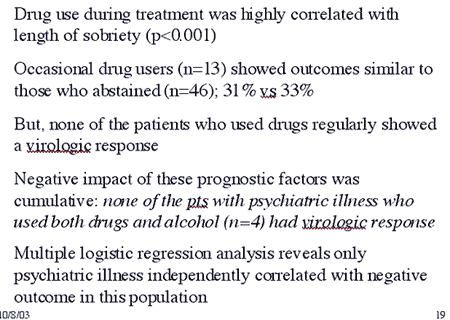
Treated patients exhibited a high prevalence of pre-existing psychiatric illness, consistent with, although somewhat higher than, previous studies which typically show comorbid psychiatric disease in approximately 40–50% of IDUs. This factor is likely due to the means by which these data were collected: self report rather than direct testing. Studies have shown that psychiatric illness has a substantial negative effect on treatment outcome, leading to recommendations that those with significant psychiatric illness should not be treated (Ho et al., 2001). However, other studies have shown that interferon treatment can be safely undertaken in patients with psychiatric disease (Pariante; Van and Van), and our study confirms these observations. Interestingly, although the dropouts in the study had a modestly higher prevalence of pre-existing psychiatric disease than the entire study population (73 vs. 62%, respectively), there was no statistically significant correlation of psychiatric illness with treatment discontinuation.
Indeed, the numbers of patients that completed treatment despite preexisting depression is remarkable. This may be due to aggressive intervention with antidepressants, since 88% of treated subjects were taking antidepressants by the end of the treatment period, likely impacting upon their ability to tolerate the interferon-mediated depression. Clearly, psychiatric illness is an important component of treatment discontinuation in MM patients, as it is in other treated populations. However, depression as a preexisting condition is substantially more common and therefore, the treated IDU has a statistically lower chance of discontinuing treatment for depression than other treated subjects. Based on our data as well as that of others showing the benefit of SSRIs in preventing interferon-mediated depression (Musselman et al., 2001), premedicating MMT patients with antidepressants should be strongly considered prior to HCV treatment.
An interesting consideration is the possibility that methadone may modulate interferon-mediated depression as well as other side effects, an assertion supported by the fact that approximately 42% of subjects in this study raised their methadone dose by a median of 10 mg during their therapy. As a potential adjunct to improving treatment adherence, this issue deserves further study.
Despite multiple barriers to HCV treatment in this population, the end-of-treatment response rate as measured by undetectable virus at the completion of treatment is similar to that of other studies in non-opioid-dependent populations, 54 versus 51%. Clearly, arguments that HCV treatment should not be undertaken in this population because of poor adherence, psychiatric disease, or altered immune status need to be reexamined in light of these results. Although these preliminary results begin to address the ongoing controversy about the appropriateness of HCV treatment in this population (Edlin and Davis), the SVR rate 6 months after treatment completion will be a more conclusive estimate of treatment outcome. Additionally, the study's lack of a comparison group and its small sample size limit firm statistical comparisons, and larger-scale study is warranted.
With limited access to liver transplantation and more advanced histologic disease, recovering IDUs are in more pressing need of timely intervention. A treatment program that is carefully tailored to the specific needs of this population, with aggressive psychiatric treatment and careful monitoring, appear to compensate for the high prevalence of negative prognostic factors such as underlying mental illness and advanced liver disease.
Further work needs to be done on improving protocols for psychiatric treatment and for improving treatment adherence, which is a major factor affecting virologic response rates. New regimens that include longer-acting pegylated interferons will offer an interesting alternative to the current treatment protocol and may further improve outcomes. The potential impact of recidivism and potential for reinfection need to be addressed in long-term studies. However, these results suggest that a decision to treat hepatitis C should not be negatively influenced by MMT despite prognostic indicators that might suggest otherwise. Indeed, our results show a more urgent need for HCV diagnosis and treatment in the MMT population.
References
Alter et al., 1999. M.J. Alter, D. Kruszon-Moran, O.V. Nainan, G.M. McQuillan, F. Gao, L.A. Moyer, R.A. Kaslow and H.S. Margolis , The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. New Engl. J. Med. 341 (1999), pp. 556–562.
Centers for Disease Control and Prevention, 1998. Centers for Disease Control and Prevention, 1998. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. 47(RR-19), 1–39.
Davis and Rodrigue, 2001. G.L. Davis and J.R. Rodrigue , Treatment of chronic hepatitis C in active drug users. New Engl. J. Med. 345 3 (2001), pp. 215–217.
Detre et al., 1997. K.M. Detre, S.H. Bell and M. Lombardero , Liver transplantation for chronic viral hepatitis. Viral Hepat. Rev. 2 (1997), pp. 219–228.
Di Bisceglie et al., 1991. A.M. Di Bisceglie, S.E. Order, J.L. Klein, J.G. Waggoner, M.H. Sjogren, G. Kuo, M. Houghton, Q.L. Choo and J.H. Hoofnagle , The role of chronic viral hepatitis in hepatocellular carcinoma. Am. J. Gastroenterol. 86 3 (1991), pp. 335–338.
Donahue et al., 1991. J.G. Donahue, K.E. Nelson, A. Munoz, D. Vlahov, L.L. Rennie, E.L. Taylor, A.J. Saah, S. Cohn, N.J. Odaka and H. Farzadegan , Antibody to hepatitis C virus among cardiac surgery patients, homosexual men, and intravenous drug users in Baltimore, MD. Am. J. Epidemiol. 134 (1991), pp. 1206–1211.
Edlin et al., 2001. B.R. Edlin, K.H. Seal, J. Lorvick, A. Kral, D.H. Ciccarone, L.D. Moore and B. Lo , Is it justifiable to withhold treatment for hepatitis C from illicit-drug users. New Engl. J. Med. 345 3 (2001), pp. 211–214.
Esteban et al., 1989. J.I. Esteban, R. Esteban, L. Viladomiu, J.C. Lopez-Talavera, A. Gonzalez, J.M. Hernandez, M. Roget, V. Vargas, J. Genesca and M. Buti , Hepatitis C virus antibodies among risk groups in Spain. Lancet 2 (1989), pp. 294–297. Abstract | Full Text + Links | PDF (614 K)
Fried et al., 2001. M.W. Fried, M.L. Shiffman, R.K. Reddy, C. Smith, G. Marino, F. Goncales, D. Haeussinger, M. Diago, G. Carosi, J.-P. Zarski, J. Hoffman and J. Yu , Pegylated (40 kDa) interferon -2a (PEGASYS™) in combination with ribavirin: efficacy and safety results from a phase III, randomized, actively-controlled multicenter study. Gastroenterology 120 Suppl. (2001), p. A-55.
Garfein et al., 1996. R.S. Garfein, D. Vlahov, N. Galai, M.C. Moherty and K.E. Nelson , Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am. J. Publ. Health 86 (1996), pp. 655–661.
Hagan et al., 2001. H. Hagan, H. Thiede, N.S. Weiss, S.G. Hopkins, J.S. Duchin and E.R. Alexander , Sharing of drug preparation equipment as a risk factor for hepatitis C. Am. J. Publ. Health 91 (2001), pp. 42–46.
Hershow et al., 1998. R.C. Hershow, L.A. Kalish, B. Sha, M. Till and M. Cohen , Hepatitis C virus infection in Chicago women with or at risk for HIV infection: evidence for sexual transmission. Sex Transm. Dis. 25 (1998), pp. 527–532.
Ho et al., 2001. S.B. Ho, H. Nguyen, L.L. Tetrick, G.A. Opitz, M.L. Basara and E. Dieperink , Influence of psychiatric diagnoses on interferon- treatment for chronic hepatitis C in a veteran population. Am. J. Gastroenterol. 96 1 (2001), pp. 157–164. Abstract | Full Text + Links | PDF (129 K)
Jamal et al., 1999. M.M. Jamal, A. Soni, P.G. Quinn, D.E. Wheeler, S. Arora and D.E. Johnston , Clinical features of hepatitis C-infected patients with persistently normal alanine transaminase levels in the Southwestern United States. Hepatology 30 (1999), pp. 1307–1311.
Manns et al., 2001. M.P. Manns, J.G. McHutchison, S.C. Gordon et al., Peginterferon -2b in combination with ribavirin compared to interferon -2b plus ribavirin for initial treatment of chronic hepatitis C: results of a randomized controlled trial. Lancet 358 (2001), pp. 958–965. Abstract | Full Text + Links | PDF (110 K)
McCarthy and Flynn, 2001. J.J. McCarthy and N. Flynn , Hepatitis C in methadone maintenance patients: prevalence and public policy implications. J. Add. Dis. 20 1 (2001), pp. 19–31.
McHutchison et al., 1998. J.G. McHutchison, S.C. Gordon, E.R. Schiff, M.L. Shiffman, W.M. Lee, V.K. Rustgi, Z.D. Goodman, M. Ling, M.D. Cort and J.K. Albrecht , Interferon -2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. New Engl. J. Med. 339 21 (1998), pp. 1485–1492.
Musselman et al., 2001. D.L. Musselman, D.H. Lawson, J.F. Gumnick, A.K. Manatunga, S. Penna, B.A. Goodkin, K. Greiner, C. Nemeroff and A. Miller , Paroxetine for the prevention of depression induced by high-dose interferon . New Engl. J. Med. 344 13 (2001), pp. 961–966.
Novick et al., 1989. D.M. Novick, M. Ochshorn, V. Ghali, T.S. Croxson, W.D. Mercer, N. Chiorazzi and M.J. Kreek , Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. J. Pharmacol. Exp. Ther. 250 (1989), pp. 606–610.
Pariante et al., 1999. C.M. Pariante, M.G. Orru, A. Baita, M.G. Farci and B. Carpiniello , Treatment with interferon-alpha in patients with chronic hepatitis and mood or anxiety disorders. Lancet 354 (1999), pp. 131–132. Abstract | Full Text +Links | PDF (53 K)
Poynard et al., 1996. T. Poynard, V. Leroy, M. Cohard, T. Thevenot, P. Mathurin, P. Opolon and J.P. Zarski, Meta-analysis of interferon randomized trials in the treatment of viral hepatitis: effects of dose and duration. Hepatology 24 (1996), pp. 778–789.
Poynard et al., 1998. T. Poynard, P. Marcellin, S.S. Lee, C. Niederau, G.S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C.F. Trepo and J. Albrecht , Randomised trial of interferon 2b plus ribavirin for 48 weeks or for 24 weeks versus interferon 2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352 9138 (1998),pp. 1426–1432. Abstract | Full Text + Links | PDF (143 K)
Reddy et al., 1999. K.R. Reddy, J.H. Hoofnagle, M.J. Tong, W.M. Lee, P. Pockros, E.J. Heathcote, D. Albert and T. Joh, Racial differences in responses to therapy with interferon in chronic hepatitis C. Hepatology 30 3 (1999), pp. 787–793.
Reichard et al., 1999. O. Reichard, H. Glaumann, A. Fryden, G. Norkrans, R. Wejstal and O. Weiland , Long-term follow-up of chronic hepatitis C patients with sustained virological response to -interferon. J. Hepatol. 30 5 (1999), pp. 783–787. Abstract | Full Text + Links | PDF (620 K)
Van Thiel et al., 1995. D.H. Van Thiel, L. Friedlander, P.J. Molloy, S. Fagiuoli, R.J. Kania and P. Caraceni , Interferon-can be used successfully in patients with hepatitis C virus-positive chronic hepatitis who have a psychiatric illness. Eur. J. Gastroenterol. Hepatol. 7 (1995), pp. 165–168.
Van Thiel et al., 1998. D.H. Van Thiel, L. Friedlander, N. De Maria, P.J. Molloy, R.J. Kania and A. Colantoni , Treatment of chronic hepatitis C in individuals with pre-existing or confounding neuropsychiatric disease. Hepatogastroenterology 45 (1998), pp. 328–330.
Weissman et al., 1977. M.M. Weissman, D. Sholomskas, M. Pottenger, B.A. Prusoff and B.Z. Locke , Assessing depressive symptoms in five psychiatric populations: a validation study. Am. J. Epidemiol. 106 3 (1977), pp. 203–214.
Zeldis et al., 1992. J.B. Zeldis, S. Jain, I.K. Kuramoto, C. Richards, K. Sazama, S. Samuels, P.V. Holland and N. Flynn, Seroepidemiology of viral infections among intravenous drug users in Northern California. West J. Med. 156 (1992), pp. 30–35.
Zeuzem et al., 2000. S. Zeuzem, S.V. Feinman, J. Rasenack, E.J. Heathcote, M.-Y. Lai, E. Gane, J. O'Grady, J. Reichen, M. Diago, A. Lin, J. Hoffman and M.J. Brunda , Peginterferon -2a in patients with chronic hepatitis C. New Engl. J. Med. 343 23 (2000), pp. 1666–1672.
|
|
| |
| |
|
|
|