| |
Pioglitazone in Lipodystrophy
|
| |
| |
Glitazones in lipodystrophy syndrome induced by highly active antiretroviral therapy
RESEARCH LETTERS
AIDS March, 2003; Vol 17 (Issue 5):770-772
Alexandra Calmya; Bernard Hirschela; Didier Hansb; Véronique L. Karsegardc; Christoph A. Meierc Divisions of aInfectious Diseases, bNuclear Medicine, and cEndocrinology, Diabetology and Nutrition, Hôpital Cantonal Universitaire, Geneva, Switzerland. The Takeda company provided pioglitazone, as well as partial funding for the laboratory and DEXA measurements.
Summary: Anti-diabetic glitazones are known to alter fat distribution in non-HIV lipodystrophy. We therefore assessed the safety and preliminary efficacy of treatment with pioglitazone for 6 months in 11 patients with lipodystrophy related to highly active antiretroviral therapy (HAART). No serious side-effects were observed. Body fat mass (total and leg) increased significantly, whereas no changes were found in the lipid profile. The good tolerance and fat redistribution observed with pioglitazone warrants a larger randomized trial in patients with HAART-related lipodystrophy.
Comments from Jules Levin: A larger European study is ongoing. Patients in this study did not have diabetes or glucose intolerance at baseline, but the insulin levels and the aIRI (ammended insulin resistance ratio), as well as other glucose post-prandial, were significantly higher at 6 months. See results below. Studies using rosiglitazone show increased cholesterol and triglycerides, but in this study pioglitazone did not show increases. Since insulin resistance increased in this study pioglitazone may present concerns for patients with insulin resistance, diabetes, or glucose intolerance. But encouragingly lipids did not increase during 6 months of this study. The ongoing European study centered in France should yield more information.
Lipodystrophy syndrome is a major problem for the long-term use of highly active antiretroviral therapy (HAART) in HIV-positive patients. No single treatment has been validated to date, and the management of this disorder is essentially empiric: the avoidance of protease inhibitors and stavudine in the regimen, treatment of metabolic disturbances, although surgery is often the only way of reversing atrophy of the face or a prominent buffalo hump. Anecdotal reports show the possible effect of growth hormone on some symptoms of fat hypertrophy, but it has to be injected and side-effects are common. More recently, the administration of recombinant leptin was shown to reverse the metabolic changes of lipodystrophy partly in HIV-negative patients. As glitazones are thought to prevent the toxic effect of protease inhibitors on adipogenesis in vitro by enhancing perixosome proliferator-activated receptor [gamma] activity, we postulated that this class of drug might be useful in the treatment of lipodystrophy in HIV-1-infected patients on HAART.
Eligible patients were HIV-positive individuals attending the HIV clinic of University Hospital Geneva with clinical lipodystrophy (including lipoatrophy), as assessed by doctors and by the patients themselves (questionnaire). The patients' ages ranged from 30 to 51 years, the mean CD4 cell count was 683 cells/mm3 (range 425-1415) and the viral load was undetectable in all patients at baseline. All patients except one received protease inhibitors, and all had been on stavudine treatment. The mean duration of the antiretroviral regimen was 3.8 years.
The study was designed as an open label prospective trial in which each patient was compared with his or her own baseline status. Pioglitazone was given at a dose of 30 mg per day for 3 months and then 45 mg a day for a further 3 months. A dual-energy X-ray (DEXA) absorbtiometry scan was performed at months 0 and 6 using the Prodigy machine (GE-lunar, Madison, WI, USA) to assess body composition. Standard and customized regional analyses were performed to quantify the fat content (expressed as a percentage) in the region of interest likely to be affected by lipodystrophic changes: mid-leg, mid-arm, truncal (in front of D12) and whole body.
Plasma concentrations of cholesterol (total and HDL-cholesterol) and triglycerides were determined using a direct enzymatic method. Plasma levels of insulin were quantified using a radioimmunoassay (Immulite, California, USA). The amended insulin resistance index (aIRI) was calculated as the ratio of insulin levels ([mu]g/l) to glucose. Leptin was measured by radioimmune assay (Linco Research, Inc., St Charles, MO, USA). Data analysis was performed on SPSS statistical software on Windows, version 9 (SPSS, Inc., Chicago, IL, USA). Values are expressed as median plus or minus the interquartile range. The Wilcoxon tests used for the comparison of metabolic and body composition values between months 0 and 6 were performed at a two-sided 5% significance level. Correlations were assessed using the Pearson test.
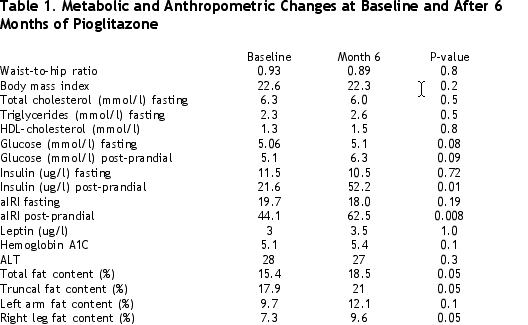
We monitored liver function tests on a monthly basis, but no significant increase was observed. All but one patient remained virologically suppressed. The values for anthropometric, metabolic measures and body composition are summarized in Table 1, below. No statistically significant changes in anthropometric measures (body mass index and waist-to-hip ratio) were found, and we noticed no significant changes in total cholesterol, triglyceride and in LDL-cholesterol values at baseline and after 6 months. None of our patients had diabetes or glucose intolerance at baseline; nevertheless, the insulin levels and the aIRI (ammended insulin resistance ratio) were significantly higher at 6 months. We observed at baseline a leptin deficiency in seven out of eight men and one out of three women, which did not significantly change at the end of the study. We found a strong correlation between baseline total fat mass and leptin level (correlation coefficient r = 0.7, P = 0.06), but the changes in body mass between months 0 and 6 were not correlated with the changes in the leptin level (correlation coefficient r = 0.19, P = 0.56).
All but one patient showed an increase in total fat mass as measured by DEXA scan after 6 months of pioglitazone treatment; indeed, the total fat content increased from a median of 15.4% (11.3-17.8) to 18.5% (12.4-20.3), which difference was significant (P = 0.05). Patient satisfaction was evaluated by questionnaire and a comparison of photographs: six out of 11 patients detected small but encouraging changes in lipoatrophic area, one experienced a significant amelioration of his physical appearance, two patients showed a continuous progression of their lipodystrophy, and two patients did not notice any changes.
Glitazones have previously been shown to improve metabolic values and to increase body fat in patients with lipoatrophic diabetes. We now show that after 66 patient-months of treatment with pioglitazones, no significant side-effect occurred in HIV-positive patients on HAART. We observed a significant increase in total body and leg fat mass. Moreover, seven of the 11 patients noticed a subjective improvement. The aIRI increased significantly after 6 months of treatment, which was associated with an increase in insulin levels after an oral glucose load. This suggests that pioglitazone did not, or only partly, prevented HAART-induced insulin resistance.
In conclusion, we found that pioglitazone treatment for a period of at least 6 months in non-diabetic HIV-positive patients on HAART was well tolerated and was associated with an increase in total body fat as well as a subjective improvement in body shape in seven out of 11 patients.
|
| |
| |
|
|
| |
| |
|
|
|