| |
Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C
|
| |
| |
Gastroenterology, May 2002, Volume 122, Number 5
Thierry Poynard*, John McHutchison‡, Michael Manns§, Christian Trepo||, Karen Lindsay¶, Zachary Goodman#, Mei–Hsiu Ling**, Janice Albrecht**, or the PEG-FIBROSIS Project Group
*Service d'Hépato-Gastroentérologie, Groupe Hospitalier Pitié-Salpêtrière, Université Paris VI, Paris, France; ‡Scripps Clinic and Research Foundation, Division of Gastroenterology/Hepatology, La Jolla, California; §Division of Gastroenterology and Hepatology, Medical School of Hannover, Hannover, Germany; ||Service d'Hépato-Gastroentérologie, Hôtel Dieu, Lyon, France; ¶Division of Gastrointestinal and Liver Disease, University of Southern California, Los Angeles, California; #Department of Hepatic and Gastrointestinal Pathology, Armed Forces Institute of Pathology, Washington, DC; and **Schering-Plough Research Institute, Kenilworth, New Jersey
ABSTRACT
Background & Aims: Liver fibrosis is an important prognostic factor in patients with hepatitis C. The effect of pegylated (PEG) interferon alone or its combination with ribavirin on fibrosis has not been established.
Methods: We pooled individual data from 3010 naive patients with pretreatment and posttreatment biopsies from 4 randomized trials. Ten different regimens combining standard interferon, PEG interferon, and ribavirin were compared. The impact of each regimen was estimated by the percentage of patients with at least 1 grade improvement in the necrosis and inflammation (METAVIR score), the percentage of patients with at least 1 stage worsening in fibrosis METAVIR score, and by the fibrosis progression rate per year.
Results: Necrosis and inflammation improvement ranged from 39% (interferon 24 weeks) to 73% (optimized PEG 1.5 and ribavirin; P < 0.001). Fibrosis worsening ranges from 23% (interferon 24 weeks) to 8% (optimized PEG 1.5 and ribavirin; P < 0.001). All regimens significantly reduced the fibrosis progression rates in comparison to rates before treatment. The reversal of cirrhosis was observed in 75 patients (49%) of 153 patients with baseline cirrhosis. Six factors were independently associated with the absence of significant fibrosis after treatment: baseline fibrosis stage (odds ratio [OR] = 0.12; P < 0.0001), sustained viral response (OR = 0.36; P < 0.0001), age < 40 years (OR = 0.51; P < 0.001), body mass index < 27 kg/m2 (OR = 0.65; P < 0.001), no or minimal baseline activity (OR = 0.70; P = 0.02), and viral load < 3.5 millions copies per milliliter (OR = 0.79; P = 0.03).
Conclusions: PEG-interferon and ribavirin combination significantly reduces the rate of fibrosis progression in patients with hepatitis C.
Background
Approximately 170 million people worldwide are infected with chronic hepatitis C virus (HCV). The degree of histologic fibrosis is an important marker of the stage of the disease because the natural history of hepatitis C involves the gradual progression of hepatic fibrosis that can eventually lead to cirrhosis. Most of the complications related to chronic infection occurs in patients who have established cirrhosis. Treatments that could halt or diminish the progression of fibrosis would theoretically be beneficial.
We have previously reported that the combination regimen of interferon and ribavirin slows progression of liver fibrosis and even leads to regression in a proportion of patients. The impact on fibrosis was related both to the response to therapy and the duration of interferon treatment.
Recently, it has been shown that the pegylated form of interferon (PEG-interferon) has a significantly higher efficacy in achieving sustained response in comparison to standard interferon. This greater efficacy has been observed both for monotherapy or in combination with ribavirin. The effect of these new regimens on histological changes has not been well characterized.
The aim of this study was to compare the efficacy of these different regimens (PEG-interferon alone or in combination with ribavirin) on fibrosis progression and on the necrosis and inflammatory features and to identify risk factors for these changes. This analysis was undertaken to determine the impact of therapy in patients who eradicate the virus, and also in patients who do not eradicate the virus during therapy.
Methods
The individual data from 4 randomized trials of PEG-interferon alfa-2b alone (Pegintron, Schering Plough, Kenilworth, NJ),8 or in combination with ribavirin, or of the combination interferon alfa-2b and ribavirin (Rebetron, Schering Plough) were obtained. Patients with serologic confirmation of chronic hepatitis C were included if they had both pretreatment and posttreatment liver biopsies. Patients were excluded if they had HBV or human immunodeficiency virus infection, a daily alcohol consumption greater than 50 g, or other forms of liver disease (hemochromatosis, autoimmune, 1-antitrypsin, steatohepatitis). In all trials, a signed informed consent was obtained after the nature and possible consequences of the studies were explained. All these pivotal trials were prospectively similarly designed with identical and centralized histologic and viral methods.
A database combining all 4 studies was created that contained the following: gender, age at first biopsy, age at infection, body mass index, presumed mode of infection (parenteral, usually intravenous drug use, transfusion, other, or unknown), type of treatment (PEG-interferon ribavirin, interferon-ribavirin, PEG-interferon, interferon), duration of treatment (24 or 48 weeks), METAVIR fibrosis stage, and activity grade at first and second biopsy, time elapsed between the 2 biopsies in months, quantification of viral load before treatment, at the end of treatment, at the end of follow-up (6 months after the end of treatment), and HCV genotype. The 10 following regimens were compared: standard interferon (alpha 2b 3 MU 3 times per week [TIW]) 24 weeks, standard interferon 48 weeks, PEG interferon 0.5 (0.5 mcg per kg) 48 weeks, PEG interferon 1.0 48 weeks, PEG 1.5 48 weeks, standard interferon and ribavirin (1000 mg, if weight <75 kg, 1200 mg >= 75 kg) 24 weeks, standard interferon-ribavirin 48 weeks, 1.5 PEG interferon 1 month then 0.5 PEG and ribavirin (1000 mg, if weight <75 kg, 1200 mg >= 75 kg) (this regimen is designated combination 0.5 PEG interferon and ribavirin), a low ribavirin (10.6 mg or less per kg), and 1.5 PEG interferon combination, and a high-dose ribavirin (more than 10.6 mg per kg) and 1.5 PEG interferon combination. The high-dose ribavirin (more than 10.6 mg per kg) and 1.5 PEG interferon combination regimen is the recommended regimen in recent European approvals because it has been shown that ribavirin was less effective when administered at a dose of 10.6 mg per kg or less.11 Of these regimens, 3 were not fully adjusted by weight: interferon monotherapy 24 and 48 weeks and combination of low-dose ribavirin and 1.5 PEG. As there was no randomized group without treatment, the first approved regimen (standard interferon alpha 2b 3 MU TIW 24 weeks) was considered as a control group and the other 9 regimens as “reinforced.”
Liver biopsies were processed using standard techniques and evaluated for stage of fibrosis and grade of activity according to the METAVIR scoring system. Fibrosis was staged on a scale of 0 to 4: F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = few septa, F3= numerous septa without cirrhosis, and F4= cirrhosis. Reproducibility of results between pathologists using this method has been established.14 Progression of fibrosis from normal liver or portal fibrosis (F0/F1) to outside the portal tract with formation of septa (F2/F3/F4) (called “significant fibrosis” throughout this article) represents a critical threshold of progressive disease caused by HCV, the last step being the constitution of cirrhosis (F4). The grading of activity, previously described15 (the intensity of necroinflammatory activity mostly based on necrosis), was scored as follows: A0 = no histological activity, A1 = mild activity, A2 = moderate activity, and A3= severe activity. One pathologist (Zachary Goodman) reviewed the biopsies at the time these individual studies were undertaken without any information concerning the clinical, biological, or treatment characteristics during the conduct of each study.
Serum HCV RNA levels had been assessed in all 4 studies, and were determined by a single central laboratory (NGI, Los Angeles, CA). A quantitative reverse-transcription multicycle polymerase chain reaction was used, with a lower limit of detection of 50 IU/mL (100 copies/mL). The median observed 3.5 millions copies/mL is equivalent to 1.300.000 IU/mL. HCV genotyping was performed as previously described.
Progression of fibrosis was analyzed by 3 methods
First, we compared the impact of the different treatment regimens on the percentage of patients who improve by at least 1 fibrosis stage, remained stable, or worsened by at least 1 stage.
Secondly, we compared the different treatment regimens according to the fibrosis progression rates per year before and after treatment. These estimates have been extensively detailed and validated previously.
The fibrosis progression rate after treatment was defined as the ratio between the difference in fibrosis stage expressed in METAVIR units between the 2 biopsies (before and after treatment) and the interval between the 2 biopsies in years. The progression rate before treatment was the ratio between the fibrosis stage in METAVIR units at the biopsy before treatment and the estimated duration of infection in years. Because of the nonnormal distribution of these estimates and the uncertainty of linearity, only medians and nonparametric tests were used. In a previous study, we had validated our estimate by comparison with paired biopsy estimates (our cohort and meta-analysis of control groups).
Third, we assessed the impact of the different treatment regimens adjusted by other risk factors in multivariate analyses. The end point was the percentage of patients with significant fibrosis at the second biopsy.
Evolution of activity (necrosis and inflammation) was analyzed by assessing for each regimen the percentage of patients who improve by at least 1-grade activity, remain stable, or worsen by at least 1 grade. We selected a 1-grade improvement in the necrosis and inflammation METAVIR scoring system as its represent a major change. One grade in METAVIR is equivalent to 4 grades in the Knodell index. This is twice the usual definition of histological improvement.
Finally, we tested the hypothesis that the reinforced regimens (9 regimens with interferon monotherapy pegylated or not for 48 weeks or combination with ribavirin) can reverse cirrhosis (change in fibrosis score based on the biopsy sample) in comparison with the “control” regimen (standard interferon alpha 2b 3 MU TIW 24 weeks which obtained only 5% of sustained virological response). If there were more reversal versus the control regimen this would strongly suggest an even more significant effect versus an absence of treatment. For this purpose, we compared the percentage of patients with baseline cirrhosis reversal between control and reinforced regimens and control regimen. To take into account the sampling error we compared the biopsy sizes between groups.
RESULTS
Of the 4493 naive patients enrolled in the 4 trials, 3010 had paired biopsies available with a 20-month mean duration between the biopsies (Table 1). There were no statistically significant differences between the original population and the population of patients with paired biopsies included in this analysis. At baseline, 2243 patients had no significant fibrosis (75%) and 767 significant fibrosis (25%), 673 (22%) had no significant activity, and 2337 (78%) significant activity. The mean weight of 1880 patients in the United States was 84 kg vs. 74 kg for the 1330 patients not in the United States (P < 0.0001).
Sustained viral response rate according to regimen
The sustained response rate varied significantly from 5% to 63% according to regimen. Sustained response rates observed in the 10 different regimens. The sustained response rate varied significantly from 5% to 63% according to regimen. 5% for interferon 24 weeks (9 of 176), 16% for interferon 48 weeks (86 of 540), 21% for PEG 0.5 (41 of 198), 27% for PEG 1.0 (48 of 178), 29% for PEG 1.5 (52 of 177), 34% for interferon-ribavirin 24 weeks (131 of 383), 51% for interferon-ribavirin 48 weeks (334 of 658), 54% for 0.5 PEG-ribavirin (194 of 361), 56% for the low ribavirin dose and 1.5 PEG combination (125 of 222), and 63% for the high ribavirin (more than 10.6 mg/kg) and 1.5 PEG interferon combination (74 of 117).
Overall histologic response
Fibrosis stage was improved in 20% of patients, stable in 65%, and worsened in 15%.
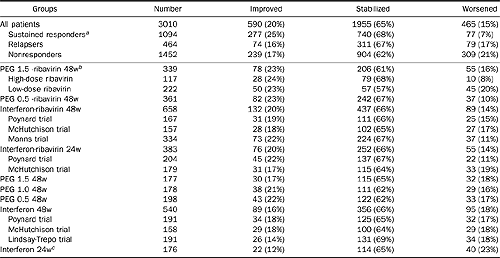
Table 2. Impact of PEG interferon and ribavirin combination regimen on fibrosis progression between baseline and posttreatment biopsies
SVRs: 25% improved fibrosis, 68% stabilized, and & worsened.
Relapsers: 16% improved, 67% stabilized, and 17% worsened.
Nonresponders: 17% improved, 62% stabilized, 21% worsened.
Rates for Peg 1.5 plus RBV high dose were better than low dose RBV. But 48 wk IFN/RBV (86%: improved+stabilized) didn’t appear worse than Peg 1.5 plus RBV (84% improved, stabilized).
|
|
| |
| |
 |
|
| |
| |
NOTE. Poynard trial has 3 arms: interferon-ribavirin 48 weeks, interferon-ribavirin 24 weeks, and interferon 48 weeks. McHutchison trials has same 3 arms plus 24-week interferon therapy. Lindsay-Trepo trial has 4 arms: PEG 1.5 48 weeks, PEG 1.0 48 weeks, PEG 0.5 48 weeks, and interferon 48 weeks. Manns trial has 3 arms: PEG 1.5 -ribavirin 48 weeks, PEG 0.5 -ribavirin 48 weeks, and interferon-ribavirin 48 weeks. There was no significant difference between randomized arms in a single trial.
aThe percentage of patients with worsening fibrosis was lower in responders vs. nonresponders (P < 0.001) and vs. relapsers (P < 0.001), as well as between relapsers vs. nonresponders (P = 0.046).
bThe percentage of patients with worsening fibrosis was lower in patients treated 48 weeks by PEG-interferon 1.5 and high-dose ribavirin (dose ribavirin greater than 10.0 mg per kg) in comparison with combination with low-dose ribavirin (P = 0.005). There were significantly fewer patients with worsening fibrosis among patients treated 48 weeks by PEG-interferon 1.5 and high-dose ribavirin combination vs. interferon 24 weeks (P = 0.001), vs. interferon 48 weeks (P = 0.02), vs. PEG interferon 0.5 48 weeks (P = 0.04), vs. PEG interferon 1.0 48 weeks (P = 0.05), vs. PEG interferon 1.5 48 weeks (P = 0.03), vs. interferon-ribavirin 24 weeks (P = 0.02), vs. interferon-ribavirin 48 weeks (P = 0.006), vs. PEG interferon 0.5 ribavirin 48 weeks (P = 0.05).
cThe percentage of patients with worsening fibrosis was higher in patients treated 24 weeks by interferon vs. interferon-ribavirin 24 weeks combination (P = 0.02), vs. interferon-ribavirin 48 weeks combination (P = 0.003) and vs. PEG-interferon 0.5 and ribavirin combination (P = 0.003) and vs. PEG-interferon 1.5 and high-dose ribavirin combination (P = 0.001).
Overall activity progression improved in 98% of SVRs, 79% of relapsers, 79% of nonresponders. Best responses in patients receiving Peg 1,5 plus high dose RBV.
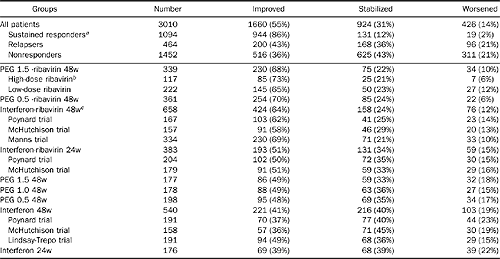
Table 3. Impact of PEG interferon and ribavirin combination regimen on activity progression between baseline and posttreatment biopsies
|
|
| |
| |
 |
|
| |
| |
NOTE. There were significant differences between randomized arms in McHutchison trial with higher percentage of patients with activity improvement in patients treated by combination interferon-ribavirin 48 weeks vs. interferon 24 weeks (P = 0.0006, vs. interferon 48 weeks P = 0.0001; combination 24 weeks vs interferon 48 weeks P = 0.006, vs. interferon 24 weeks P = 0.03). There were significant differences between randomized arms in Poynard trial with higher percentage of patients with activity improvement in patients treated by combination interferon-ribavirin 48 weeks vs. interferon 48 weeks (P < 0.001) and vs.combination 24 weeks (P = 0.02); combination 24 weeks vs. interferon 48 weeks, P = 0.007.
aThe percentage of patients with activity improvement was higher in responders vs. nonresponders (P < 0.001) and vs. relapsers (P < 0.001), as well as between relapsers vs. nonresponders (P = 0.009).
bThe percentage of patients with activity improvement was higher in patients treated 48 weeks by PEG-interferon 1.5 and high-dose ribavirin combination vs. interferon 24 weeks (P < 0.0001), vs. interferon 48 weeks (P < 0.0001), vs. PEG interferon 0.5 48 weeks (P < 0.0001), vs. PEG interferon 1.0 48 weeks (P < 0.0001), vs. PEG interferon 1.5 48 weeks (P < 0.0001), vs. interferon-ribavirin 24 weeks (P < 0.0001).
cThe percentage of patients with activity improvement was higher in patients treated 48 weeks by interferon and ribavirin combination vs. interferon 24 weeks (P < 0.0001), vs. interferon 48 weeks (P < 0.0001), vs. PEG interferon 0.5 48 weeks (P < 0.0001), vs. PEG interferon 1.0 48 weeks (P = 0.0003), vs. PEG interferon 1.5 48 weeks (P = 0.0001), vs. interferon-ribavirin 24 weeks (P < 0.0001).
Most of the differences were a 1 stage change: 16% 1 stage and 4% 2 or 3 stages for those biopsy pairs that improved; 12% 1 stage, and 3% 2 or 3 stages for those that worsened. The activity grade improved in 55%, remained stable in 31%, and worsened in 14%. At the second biopsy, cirrhosis was observed in 175 patients (6%) of 2834 patients treated with reinforced regimens and in 18 of 176 patients treated with control regimen (10%; P = 0.03).
Histologic response according to virologic response
Among patients who achieved a virologic sustained response, there was less frequently worsening of fibrosis (7%) in comparison with relapsers (17%) or nonresponders (21%) (P < 0.001 for both comparisons; Table 2), as well as more activity improvement (86% vs. 43% and 36%, P < 0.001 for both comparisons, respectively; Table 3). When relapsers were compared with nonresponders, the differences were also significant (P = 0.046 and P = 0.009, respectively).
Histologic response according to regimen
Between randomized different treatment arms there was a significant difference in 2 separate trials for activity grade improvement in favor of interferon–ribavirin 48 weeks in comparison with interferon alone 48 weeks or 24 weeks (Tables 2 and 3). There was no significant difference between randomized arms for fibrosis.
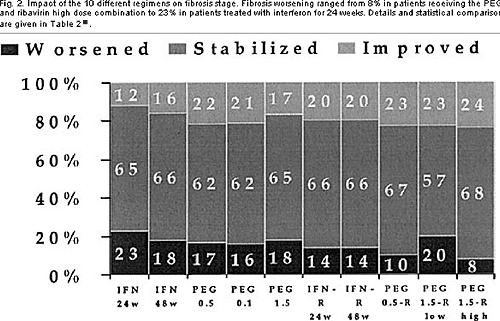
Between regimens there were also highly significant differences. Fibrosis worsening ranged from 8% in patients receiving the PEG 1.5 and ribavirin high-dose.
Fig. 2. Impact of the 10 different regimens on fibrosis stage. Fibrosis worsening ranged from 8% in patients receiving the PEG 1.5 and ribavirin high dose combination to 23% in patients treated with interferon for 24 weeks. Details and statistical comparisons are given in Table 2.
|
|
| |
| |
 |
|
| |
| |
combination to 23% in patients treated with interferon for 24 weeks (Table 2 and Figure 2).
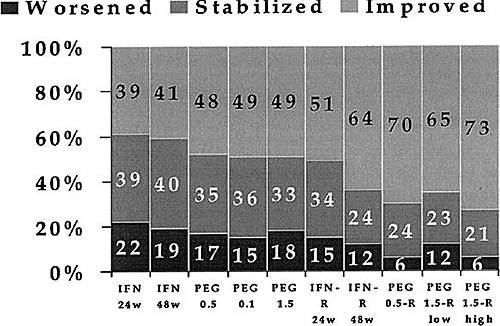
Activity improvement ranges from 73% in patients receiving the PEG 1.5 and high-dose ribavirin combination to 39% in patients treated by interferon 24 weeks (Table 3 and Figure 3).
Fig. 3. Sustained response rates observed in the 10 different regimens. Activity improvement ranges from 73% in patients receiving the PEG 1.5 and high dose ribavirin combination to 39% in patients treated with interferon 24 weeks. Details and statistical comparisons are given in Table 3
|
|
| |
| |
 |
|
| |
| |
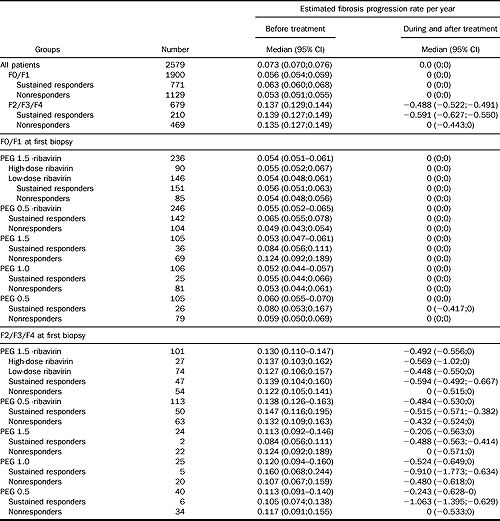
Comparison between fibrosis progression rates per year before and after treatment
All rates were lower after treatment than before both in responders and in nonresponders (all P < 0.001; Table 4).
Table 4. Impact of PEG-interferon and ribavirin combination regimen on fibrosis progression rate
|
|
| |
| |
 |
|
| |
| |
NOTE. All rates were lower after treatment than before (all Wilcoxon signed-rank test for difference in medians P < 0.001). There was no significant difference between treatments (Kruskal–Wallis variance analysis P = 0.48). There was significant difference between responders and nonresponders (P < 0.001). Impact was analyzed according to treatment regimen, baseline fibrosis stage and virologic response, in naive patients with known duration of infection.
There were no significant differences between different treatments (P = 0.48). There was a significant difference between responders and nonresponders (P <0.001).
Factors associated with the absence of significant fibrosis at the end of follow-up in multivariate analysis
Six factors were independently associated with the absence of significant fibrosis after treatment: baseline fibrosis stage, sustained viral response, age younger than 40 years, body mass index lower than 27 kg/m2, no or mild baseline activity, and viral load lower than 3.5 millions copies/mL.
Factors associated with histological improvement in patients without sustained virologic response
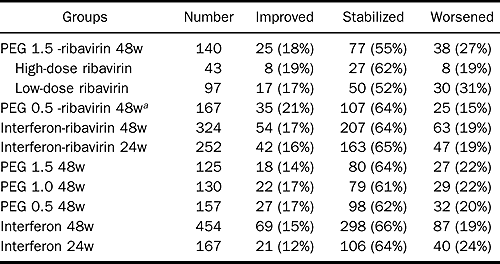
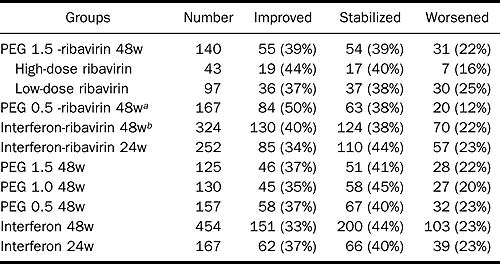
The same risk factors were associated with significant fibrosis in patients without sustained virologic response (relapsers and nonresponders). In comparison with the other regimens, PEG 0.5 and ribavirin combination had a better impact on fibrosis and on activity: 21% had demonstrable fibrosis improvement vs. 12% for interferon 24 weeks (P = 0.04) and vs. 15% for interferon 48 weeks (Table 6); 50% improvement of activity vs. interferon 24 weeks (37%, P = 0.02), vs. interferon 48 weeks (33%, P = 0.0001), vs. PEG interferon 0.5 for 48 weeks (35%, P = 0.02), vs. PEG interferon 1.0 48 weeks (35%, P = 0.007), vs. PEG interferon 1.5 48 weeks (37%, P = 0.02), vs. interferon-ribavirin 24 weeks (34%, P = 0.0008), and vs. interferon-ribavirin 48 weeks (40%, P = 0.03; Table 7).
Patients who received Peg 1.5- RBV 48 weeks: 73% improved or stabilized fibrosis, 27% worsened; 18% improved, 55% stabilized. Patients receiving high-does RBV 81% improved or stabilized; 19% worsened; 19% improved.
There was a significant association between fibrosis and activity changes but with low concordance rates in all patients. This was also observed in nonresponders (data not shown).
Table 6. Impact of PEG Interferon and Ribavirin Combination Regimen in Patients Without Sustained Virologic Response (Nonresponders or Relapsers) on Fibrosis Progression Between Baseline and Posttreatment Biopsies
|
|
| |
| |
 |
|
| |
| |
Table 7. Impact of PEG Interferon and Ribavirin Combination Regimen in Patients Without Sustained Virologic Response (Nonresponders or Relapsers) on Activity Progression Between Baseline and Posttreatment Biopsies
|
|
| |
| |
 |
|
| |
| |
Treatment of patients with cirrhosis
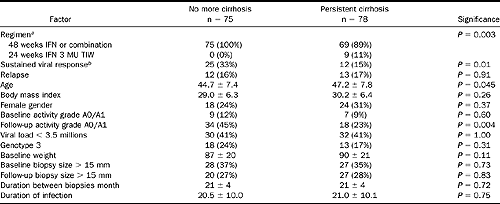
A total of 153 patients had cirrhosis at the time of the first biopsy before treatment. The “reversal” of cirrhosis (change in fibrosis score based on the biopsy sample) was observed in 75 patients (49 %), none in the control regimen (Table 8).
Table 8. Treatment of 153 patients with baseline cirrhosis: Factors associated with change in fibrosis score based on the biopsy sample (cirrhosis reversal)
One third of patients with cirrhosis reversal were sustained responders; they were also younger and had significant improvement in the activity grade in comparison to patients without cirrhosis reversal. The size of the biopsies (at baseline or at the second biopsy) were not different between patients with or without cirrhosis reversal. Among the 75 patients with cirrhosis reversal, the second biopsy sample was graded stage 3 in 23 patients, stage 2 in 26, stage 1 in 23, and no fibrosis in 3. The mean fibrosis score at the second biopsy was 1.9 ± 0.9 (SD), and the mean activity grade improved from 2.5 ± 0.7 to 1.6 ± 0.9 (P < 0.01). SEE in DISCSSUSSION below.
|
|
| |
| |
 |
|
| |
| |
aStandard interferon 3 million units 3 times per week for 24 weeks was considered as the control regimen and the other regimen as reinforced regimen.
bIn multivariate logistic regression analysis, only sustained response was significantly associated with cirrhosis reversal (odds ratio = 0.39; 95% confidence interval, 0.17–0.85; P = 0.02).
DISCUSSION
This overview of 4 pivotal randomized trials has permitted us to assess the incremental benefit of 10 different regimens on the histological features of patients infected with hepatitis C virus. These regimens given for 24 or 48 weeks allowed us to observe an improvement of necrosis and inflammation grades and a reduction of fibrosis progression at least during the 2 years' histological follow-up. This analysis has also demonstrated that the histological improvement was related both to the viral response and to several baseline factors. The combination of PEG-interferon and ribavirin eradicates the virus in more than 50% of patients, and therefore potentially can prevent cirrhosis, amplifying our previous results with standard interferon plus ribavirin combination. Also, half of the patients with baseline cirrhosis treated with the reinforced regimen had a disappearance of cirrhosis at the time of the subsequent follow-up biopsy.
Compared with our prior analyses, a new factor, the body mass index, was strongly associated with fibrosis progression even after adjustment for the 3 main factors previously identified, viral response, fibrosis stage, and age. Gender was probably a confounding factor, which disappears when body mass index was taken into account. Alcohol consumption was not assessed during the treatment and follow-up periods in all of these trials. This is a limitation of the present study. High alcohol consumption was an exclusion criterion, but changes in alcohol consumption may have influenced fibrosis progression. However, the regimens were randomized, which precluded a major risk of bias.
This analysis combining individual data has 3 main methodological weaknesses: (1) paired biopsy was not available for all included patients; (2) the absence of direct comparisons by randomization of the 10 different regimens in a single prospective trial; and (3) the absence of a control group without treatment. A bias related to patient's selection could be reasonably excluded, as there were no different characteristics between all patients randomized versus patients who had paired biopsies. The direct comparisons by randomization of the 10 different regimens in a single prospective trial were in practice impossible.
Each new trial included in its design the previous standard regimen. The PEG interferon monotherapy trial was started in 1997, before the end of the standard interferon-ribavirin combination trials in 1998. Despite the absence of randomization between the 10 regimens, a randomization was always performed versus the standard treatment at this time. Furthermore, the pooling of data was possible because all the 4 trials were designed prospectively together with centralized endpoints, and all known prognostic factors were taken into account in univariate and multivariate analyses. The individual data from the first trials comparing interferon monotherapy versus controls without treatment were not available. However, the first approved regimen (interferon 3 MU TIW for 24 weeks) had already an impact on the natural history of chronic hepatitis C as demonstrated in meta-analysis of randomized trials21 and in historical or randomized comparisons. Among 70 nontreated patients (when interferon was not available) with 2 biopsies assessed retrospectively we observed an improvement of activity in 17% and a worsening of fibrosis in 64% of patients. In the present study, interferon monotherapy for 24 weeks was associated with an improvement of activity grade in 37% and a worsening of fibrosis stage in 24% of treated patients. Even if in a conservative approach this first approved regimen was considered as the control regimen group, very significant breakthroughs have been obtained thereafter. The last approved regimen, the PEG-interferon and high-dose ribavirin combination, had the highest histological benefit ever observed: an activity improvement was observed in 73% of patients and a worsening of fibrosis stage was observed in only 8%.
The different regimens were more and more effective on viral response with 63% of sustained response with the PEG 1.5 high-dose ribavirin combination that is 58% more than the 5% achieved with the first approved regimen. When a sustained response was achieved, the histological impact was the same whatever the regimen: activity improvement was observed in 86% of patients with only 7% of patients with worsening fibrosis.
The percentage of patients with reversal of cirrhosis after treatment was surprisingly high among patients receiving the reinforced regimen (52%). Before a generalized conclusion that cirrhosis can be cured, several limitations of our study must be underlined. Cirrhosis reversal was defined as a change in fibrosis score based on the biopsy sample. A sampling error is possible especially between stage 3 (extensive fibrosis) and stage 4 (cirrhosis), particularly with biopsies of small size. However, there was no difference in biopsy sizes between patients with cirrhosis reversal and those whose biopsies did not change. If we analyzed stage 3 and stage 4 together, there was also a very significant improvement rate in fibrosis in 50% of cases (data not shown). One concordant fact is the association between activity improvement and cirrhosis reversal: twice as many patients had none or mild necroinflammatory features at the follow-up biopsy in comparison with patients who still had cirrhosis. A sampling error cannot explain the significant association between cirrhosis reversal and virologic response and activity improvement. The patients with cirrhosis reversal were younger, and it is possible that early cirrhosis is easier to reverse than more established cirrhosis. These large prospective studies with repeated biopsies have finally identified a new category of extensive fibrosis that could be named a “reversible cirrhotic stage.” Because the follow-up in these trials was less than 48 weeks after the end of the treatment, the possibility of a continued decrease in fibrosis among patients with a sustained virologic response should be evaluated with long-term histologic and virologic follow-up studies. Recent noninvasive biochemical markers of fibrosis may also be useful.
In virologic nonresponders, there was less fibrosis regression and more fibrosis progression than in responders. However, it may be incorrect to conclude that the combination regimen or interferons alone are valueless in virologic nonresponders. By comparing the fibrosis progression rate of virologic nonresponders after interferon to their estimated progression before treatment and to nonrandomized matched untreated patients, we and other investigators have previously observed that interferon slowed the natural fibrosis progression observed before treatment (although the impact was weaker than in sustained responders).
This has been confirmed by a randomized trial for necrosis and inflammation. In the present study, we also observed a reduction of fibrosis progression rates in comparison to estimates before treatment, especially among patients with initial significant fibrosis. Because there is no certainty concerning the linearity of the fibrosis progression, we used only medians and nonparametric methods for all comparisons. It seems unfair not to take into account information based on 17 years of disease evolution. The fibrosis stage at the first biopsy for each patient represents an excellent abstract of this balance between extracellular matrix formation and degradation. One weakness of the fibrosis progression rate estimated during and after treatment is the short time elapsed between biopsies with a mean of 20 months. This argues against the concept of stopping treatment too early in patients without a sustained virologic response. The antifibrotic and histologic efficacy of these regimens should be carefully considered before prematurely stopping treatment, especially in patients who have significant fibrosis.
In a patient with a rapid fibrosis progression rate, it may be clinically relevant to prevent fibrosis progression and cirrhosis complications even if the virus is still detectable. The retrospective analyses of these randomized trials suggested 2 options for maintenance therapy, either PEG-1.0 monotherapy which is the simplest and well-tolerated regimen or the combination PEG-0.5-ribavirin, which was the most effective regimen in nonresponders on necrosis and inflammation. These options should be validated prospectively.
This study permitted us to identify body mass index as a major factor associated with significant fibrosis even in treated patients. There are at least 2 factors explaining this association. First, the viral efficacy of both interferon and ribavirin is related to a correct dosage adjusted on the patient's weight. Secondly, there is a strong relationship between fibrosis progression and metabolism. Recently, we observed that the liver fibrosis risk was increased in overweight patients particularly when the body mass index was greater than 27.28 Since the beginning of these pivotal studies the mean weight is increasing particularly in patients in the United States. The percentage of patients in the United States with a body mass index greater than 27 was 43% in the first combination trial (1995), 51% in the second (1997), and 57% in the last (1998). In a trial of patients not in the United States, these percentages were 28%, 28%, and 32%, respectively.
We conclude that the combination of PEG interferon-ribavirin has the potential to reduce the morbidity and mortality of chronic hepatitis C by reducing fibrosis progression and the incidence of cirrhosis. This effect was most prominent in patients who achieved a virologic response, which is best achieved by the combination of PEG-interferon and high-dose ribavirin. Independent of achieving a sustained viral response to treatment, patients without extensive fibrosis at baseline, younger than 40 years of age, and with body mass index lower than 27 had a much lower progression of liver fibrosis. Cirrhosis reversal seems possible in patients with chronic hepatitis C.
Editorials Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C
Michael J. P. Arthur Liver Group Division of Infection, Inflammation, and Repair University of Southampton Southampton, England. Professor Arthur is a consultant to Glaxo-Smith-Kline and to Cambridge Antibody Technology.
Chronic hepatitis C virus (HCV) infection poses a major worldwide health care problem as a leading cause of chronic liver disease and cirrhosis. The burden of disease in the United States and in Europe is substantial, and HCV infection is now the most common underlying diagnosis in patients listed for liver transplantation. The liver disease is usually insidious in onset and most of the morbidity and mortality occur as a direct consequence of the development of liver fibrosis and cirrhosis and later complications. In the majority of patients, a striking feature of this disease is the relatively slow rate of progression of the liver fibrosis, with significant problems usually developing after an interval of 15–20 years or longer.
Fortunately, the last decade has witnessed major improvements in our ability to treat this disease, with progressive improvements in the rates of viral clearance accompanied by normalization of transaminases and improvements in necro-inflammatory scores on liver histology. For some time, a key question has been whether treatments that lead to viral clearance can influence the rate of progression of liver fibrosis and perhaps in some cases lead to regression of this complex pathologic process. The article in this issue of GASTROENTEROLOGY by Poynard et al.1 analyzes the results of 4 previous major clinical trials involving 3010 patients that were randomized to various treatment regimes with either interferon or pegylated interferon, with or without the addition of ribavirin (a total of 10 different regimens are analyzed), and that had both pre- and post-treatment liver biopsies. This report extends a previous similar study of 1509 chronic HCV patients6 but concentrates more on the effects of antiviral treatment on patients with established cirrhosis. The observations in the new study by Poynard et al. are very important and provide clear evidence for major beneficial effects of antiviral therapy on liver fibrosis; put simply, the more effective the treatment regime in terms of clearance of HCV, the more likely there is to be a decrease in severity of liver fibrosis. The most striking result was the finding that of a total of 153 patients with cirrhosis, reversal was observed in 75 (49%).
Do I hear you say “But that's impossible, cirrhosis isn't reversible!” If so, this paper and a summary of other studies in the field may help to change your mind. There is now a substantial body of evidence in both human liver disease and in animal models to indicate that liver fibrosis and cirrhosis are dynamic processes that can both progress and regress over time, depending in part on whether or not the underlying cause is persistent.
But first, are we sure that the observations of Poynard et al. are correct, or could there have been any confounding factors? These could theoretically include the sampling error of liver biopsy, variable pathological interpretation of the same liver biopsy (intraobserver variation), or overenthusiastic interpretation of minor changes in liver fibrosis scores. All of these problems were considered and addressed in the study. Sampling error is a perennial problem, but is less likely to be relevant in a disease such as HCV if liver biopsy specimens are of an adequate size. Table 1 in the report indicates that liver biopsy size ranged from 14 to 16 mm in each of the treatment regimes, which most pathologists would consider to be more than adequate. One highly experienced pathologist, blinded to knowledge of the treatment regime, using the METAVIR scoring system, assessed all liver biopsy specimens. This methodology has been reported to yield a high degree of reproducibility in the assessment of fibrosis.7 Moreover, the vast numbers of patients under analysis would mean that errors caused by sampling or inconsistent scoring of liver fibrosis are likely to be equally distributed across groups. The numeric score obtained in the METAVIR system is a qualitative and descriptive measure of a histologic appearance, not a quantitative assay of the extent of liver fibrosis. The difference between grades must therefore be interpreted accordingly. For example, grade 4 does not mean that there is twice as much fibrosis as grade 2. The difference in the amount of hepatic collagen between these 2 METAVIR grades would be manifold. This potentially leads to problems with interpretation because a change of 1 grade at the top end of the range (grade 4 to 3) could represent a far greater change in the quantity of fibrotic matrix in the liver than at the lower grades (e.g., changing from grade 2 to 1).
The authors have addressed these difficulties by looking at their data in a number of different ways to determine if the same trends are observed with each approach. First, they have looked at the proportion of patients in each treatment group that have improved, deteriorated, or remained unchanged in the extent of liver fibrosis (see Figure 2 in the article). For the regime with the worst sustained viral clearance rate (of 5% on interferon only for 24 weeks), 12% of patients had an improved fibrosis score, 65% remained unchanged, and 23% deteriorated. In contrast, the results for patients with the highest sustained viral clearance rates (of 63% on pegylated interferon and high-dose ribavirin) were 24% improved, 68% unchanged, and 8% with deterioration. Another method of examining the data was to compare the calculated fibrosis progression rate before and after each treatment. This is perhaps the most controversial aspect of the study because the fibrosis progression rate before treatment is calculated by dividing the METAVIR fibrosis score at the time of the initial biopsy by the estimated years of duration of infection, whereas the post-treatment rate is calculated by looking at the difference in the METAVIR score between the pre- and post-treatment liver biopsies divided by the interval (in years). Both estimates assume linearity, which may not be correct. In the analyzed studies, the median time between pre- and post-treatment liver biopsies was only 20 months. Because many patients were unchanged in their fibrosis score, the calculated post-treatment fibrosis progression rate was 0 in a significant proportion. This highlights the difficulty of comparing the pre- and post-treatment fibrosis progression rates. Notwithstanding these concerns, the overall results (Table 4 in the report) showed the same interesting trends, with a reduction in the rate of progression of liver fibrosis after treatment of HCV and the greatest effect seen in patients with sustained viral clearance. For patients with METAVIR fibrosis stages 2, 3, and 4 at initial biopsy, the fibrosis progression rate before treatment was 0.137 compared with –0.488 after treatment. In the same group of patients, fibrosis progression rates after treatment were significantly lower in patients that cleared HCV (–0.591) than in those with viral persistence (0.0).
The third method of analysis used by the authors was to test the hypothesis that the reinforced regimes (all 9 regimes with interferon monotherapy or not for 48 weeks or combination with ribavirin) can reverse cirrhosis in comparison with the control regimen (of standard interferon monotherapy for 24 weeks only). In the control group there were no cases of an improvement in cirrhosis, which compared with 75 of 153 cases (49%) in the reinforced regimes. Of those that had an improvement in cirrhosis, this was by 1 stage (to stage 3) in 23 patients, by 2 stages (to stage 2) in 26, by 3 stages (to stage 1) in 23, and by a remarkable 4 stages (i.e., no remaining liver fibrosis) in 3.
These are very important results because they show that human liver fibrosis and cirrhosis can reverse to a very significant degree histologically, particularly if there is treatment-induced clearance of HCV. The implication is that this will be associated with improvements in long-term clinical outcome in terms of morbidity and mortality, but such data are not yet reported. There is a major need for the patients in the studies reported by Poynard et al. to be followed for longer periods to determine if the improvements in liver fibrosis continue to accrue, particularly in those that have cleared HCV, and to determine if there are long-term beneficial effects on clinical outcome. It would also be very interesting to know if there is an associated measurable reduction in portal pressure in patients in whom there is a documented improvement in liver fibrosis and cirrhosis, particularly in those with documented cirrhosis and portal hypertension at the start of antiviral therapy.
The suggestion that liver fibrosis and cirrhosis are reversible is not a novel concept, but large-scale clinical trials in the treatment of chronic hepatitis C are making this concept more widely known and generally acceptable, thus helping to complete an important paradigm shift in hepatology. Earlier reports of reversibility of liver fibrosis and cirrhosis have tended to be smaller in scale but included a wide range of different liver diseases. A common theme in all of these reports is an effective therapeutic intervention that either removes or effectively suppresses the underlying disease process. Reversibility of cirrhosis was reported over 30 years ago in patients with hemochromatosis after long-term intensive venesection therapy.8 Improvements in liver fibrosis and cirrhosis have also been reported in patients with autoimmune chronic active hepatitis and primary biliary cirrhosis11 after effective immunosuppressive therapy. There is also clear evidence of reversibility of liver fibrosis in patients with chronic hepatitis B virus infection after effective suppression of viral replication with lamivudine and in chronic hepatitis D virus infection after treatment with long-term interferon. More recently, reversibility of liver fibrosis and cirrhosis has been reported in patients that have undergone surgical biliary decompression to relieve chronic bile duct obstruction.14 The diversity of diseases and the different nature of the interventions in these reports serve to indicate that we are witnessing a generically applicable and important phenomenon in the liver's response to injury and in its ability to undergo regeneration and repair. An understanding of the cell and molecular mechanisms involved in the process of reversibility of liver fibrosis are therefore of major importance and may point the way forward to new therapeutic strategies that attempt to mimic or promote this process.
Liver fibrosis is characterized by proliferation and activation of hepatic stellate cells (HSCs), which develop a myofibroblastic phenotype and are the main cellular source of the fibrillar collagens and other matrix proteins that accumulate in chronic liver disease. The fate of these cells and the mechanisms by which fibrotic matrix is removed have been studied in animal models of reversibility of liver fibrosis. These studies have shown that activated HSCs rapidly undergo apoptosis after 4 weeks of CCl4 liver injury, if the stimulus is withdrawn. Apoptosis of activated HSCs was also observed after decompressive surgery in the bile duct ligation model of liver injury. In the regression phase, fibrotic liver matrix is degraded and removed as a consequence of the action of metalloproteinases, including MMP-1, MMP-2, and MMP-14, and this occurs in part because of a rapid decrease in expression of metalloproteinase inhibitors, such as TIMP-1. There is significant interplay between these various events because TIMP-1 has recently been shown to be antiapoptotic for activated HSCs, an effect that was mediated via the MMP inhibitory effects of this molecule. Apoptosis of activated HSCs is also decreased in mice that are transgenic for mutated collagen I that is resistant to the effects of MMPs. These studies indicate that the key events in the regression of liver fibrosis are interlinked and include apoptosis of HSCs, decreased expression of TIMP-1, increased MMP activity, increased degradation of collagen I, and altered HSC-matrix interactions. These events combine to further promote apoptosis of activated HSCs. As yet, it is not known which of these events is the primary regulatory factor, but these observations have opened up the prospect of novel therapeutic strategies for the treatment of liver fibrosis by trying to initiate regression via these pathways.
It is likely that this sequence of cell and molecular events is operative during reversal of liver fibrosis and cirrhosis after treatment for HCV, but as yet there are very few studies of such mechanisms in human disease. There are data consistent with this suggestion in patients with chronic HBV infection treated with lamivudine. Serial biopsies performed in these patients demonstrated that expression of smooth muscle actin (an indirect measure of the number of activated HSCs) was decreased as liver fibrosis improved after lamivudine treatment.
Many key questions remain with regard to the reversibility of human liver fibrosis and cirrhosis. For example, can disease regress to the extent that the liver returns to complete normality? The Poynard et al. report suggests that this occurred in 3 patients who had established cirrhosis on their first biopsy. Experimental animal evidence suggests that some fibrosis may persist for very long periods after withdrawal of liver injury, particularly if the remaining collagen is cross-linked (Iredale JP, personal communication, October 2001) and thus more resistant to the action of metalloproteinases. Against this background, it is interesting that Poynard et al. found that cirrhosis was more likely to reverse in younger patients, which led them to suggest that early cirrhosis may reverse more readily than established disease. A knowledge of the way in which cirrhosis can change during regression may also be important because there is evidence to suggest that cirrhosis changes from a micronodular to a macronodular type as it regresses.21 Clearly, the macronodular phase of regressing cirrhosis in a patient with HCV could be difficult to diagnose on a single repeat liver biopsy. The case for complete resolution of cirrhosis is therefore not yet made conclusively in human liver disease, but the study of Poynard et al. challenges all of us to accept that the traditional view of cirrhosis as a progressive irreversible disease is no longer correct. This augurs well for the future of our patients with chronic HCV infection, particularly if they clear the virus with treatment. This study also encourages further scientific investigation of the key cell and molecular mechanisms of liver fibrosis and raises the prospect that an antifibrotic agent aimed at promoting regression of disease may be an important therapeutic strategy for the future.
|
|
| |
|
|
|