 |
 |
 |
| |
LdC, new HBV Drug: 4-week Dosing, Safety & Antiviral Efficacy Study
|
| |
| |
Reported by Jules Levin
"VALTORCITABINE PROVIDES POTENT SUPPRESSION OF HEPATITIS B VIRUS IN PATIENTS WITH CHRONIC HEPATITIS B: RESULTS OF A PHASE I/II CLINICAL TRIAL"
Ching L Lai, University of Hong Kong, Hong Kong, Hong Kong Special Administrative Region of China; Nathaniel A Brown, Maureen Myers, Idenix Pharmaceuticals, Cambridge, MA; Man F Yuen, University of Hong Kong, Hong Kong, Hong Kong Special Administrative Region of China; Chun T Wai, National University Hospital, Singapore, Singapore; Deborah Lloyd, Keith Pietropaolo, Xiao J Zhou, George Chao, Idenix Pharmaceuticals, Cambridge, MA; Seng G Lim, National University Hospital, Singapore, Singapore
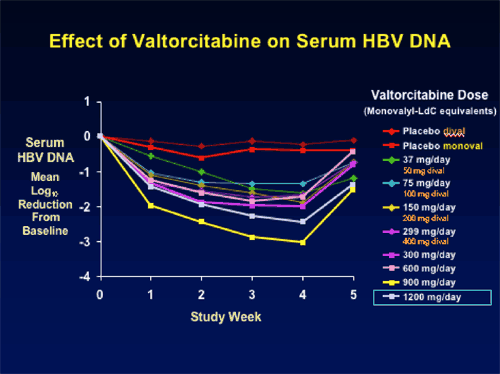
BRIEF SUMMARY: 50 Asian patients, mostly men who were HBsAg & HbeAg positive were randomized blindly to various doses of LdC 50 to 1200 mg once daily. HBV DNA suppression with valtorcitabine was greatest at week 4 with the 900 mg dose: mean 3.04 log10. Investigators reported satisfactory safety in all patient cohorts: no pattern of adverse events or laboratory abnormalities related to treatment, dose, or time. Investigators concluded that the clinical and pre-clinical efficacy and safety data support the potential utility of valtorcitabine as a component of combination therapy. Exploratory phase IIb trial of valtorcitabine + telbivudine combination therapy to begin soon.
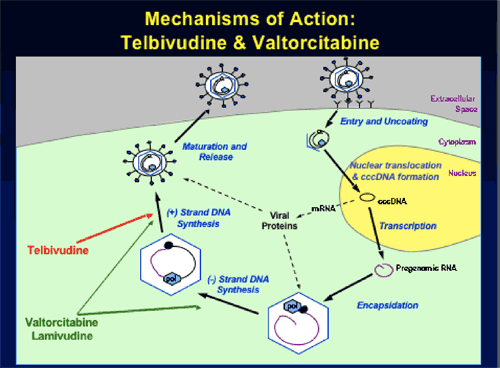
HBV-Specific L-Nucleosides: Telbivudine (LdT) and Valtorcitabine (val-LdC)
--Small-molecule HBV polymerase inhibitors
--HBV-specific
--High intracellular triphosphate concentrations
--Once daily oral dosing
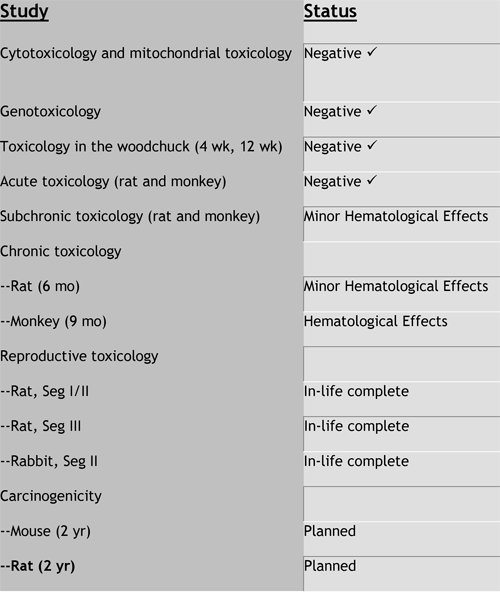
--Favorable preclinical toxicology
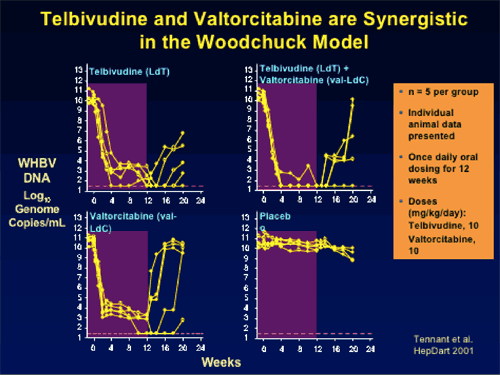
--Marked viral load reduction (8-10 log10) in woodchuck HBV model
Idenix plans to explore combination therapy of LdT+LdC.
Rationale for Telbivudine + Valtorcitabine Combination Therapy
--Potentially complementary mechanisms of action on 1st and 2nd-strand HBV DNA synthesis
--Combination has synergistic antiviral activity in vitro and in woodchuck HBV model
--Both compounds have excellent safety profiles
Telbivudine phase III clinical trials are ongoing.
The presentation at AASLD is an assessment of the clinical safety & antiviral efficacy of LdC or valtorcitabine.
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
Valtorcitabine Preclinical Toxicology
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
Phase I/II Clinical Trial of Valtorcitabine
Study design
1. Randomized, double-blind, placebo-controlled dose escalation trial of valtorcitabine:
-- 50 -- 1,200 mg/day (7 dosing cohorts)
-- Once daily treatment for 4 weeks, with 12 weeks' follow-up.
-- 7 pts per dose group, randomized 6:1 to valtorcitabine or placebo
2. HBeAg positive adult patients with compensated chronic hepatitis B:
-- Serum HBV DNA >107 copies/mL; serum ALT 1-5 xULN
3. 2 clinical sites: SG Lim (Singapore) and C-L Lai (H.K.)
4. First 4 dosing cohorts treated with divalyl ester prodrug of LdC
5. Change to monovalyl ester of LdC (more stable) for higher dose cohorts
6. Last dose of divalyl LdC repeated with monovalyl equivalent to provide continuity and evidence of similar bioavailability
BASELINE CHARACTERISTICS
Mean age from 27 to 31 yrs.
Mostly males
HBV DNA (median log copies/ml: 7.1 to 9.3
All patients were ethnically asian
All patients were HBsAg positive & HBeAg positive at baseline.
CHANGES IN HBV DNA
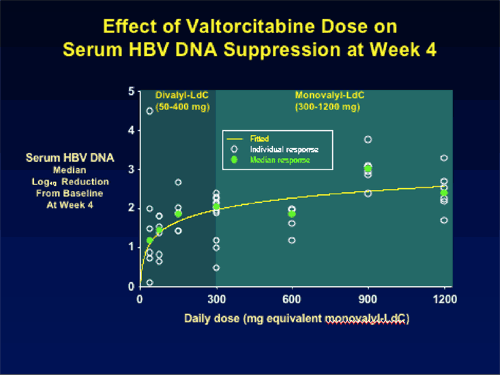
Seven of the eight dose cohorts have completed treatment. Consistent, dose-related HBV DNA reductions have been observed, ranging from a mean 1.63 log10 for the 50 mg/day group at Day 28, to 3.04 log10 copies/mL for the 900 mg/day. The final dose cohort, 1200 mg/day showed less of a reduction in HBV DNA at week 4 of -2.2 log copies/mL. ,
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
|
| |
|
 |
 |
|
|