 |
 |
 |
| |
Durability of SVR, 5 Year follow-up Pegasys plus Copegus
|
| |
| |
Reported by Jules Levin
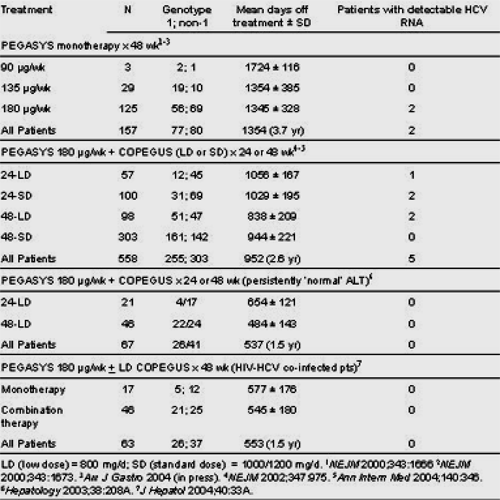
This study was reported at the AASLD conference just completed and shows from putting together from numerous Pegasys/Copegus studies that >99% of patients with SVR have maintained SVR for up to 5 years, as long as study has followed these patients so far. If you look at the table below you'll see some description of the 5/558 relapsers.
"DURABILITY OF SUSTAINED VIROLOGICAL RESPONSE (SVR) AFTER TREATMENT WITH PEGINTERFERON ALFA-2A (40KD) (PEGASYS®) ALONE OR IN COMBINATION WITH RIBAVIRIN (COPEGUS): RESULTS OF AN ONGOING LONG-TERM FOLLOW-UP STUDY"
Authors: Mark Swain, University of Calgary, Calgary, AB, Canada; Ming-Ying Lai, National Taiwan Hospital, Taipei, Taiwan Republic of China; Mitchell L Shiffman, Virginia Commonwealth University Health System, Richmond, VA; Graham E Cooksley, Royal Brisbane Hospital, Herston, Australia; Armand Abergel, Hopital Hotel Dieu, Clermont-Ferrand, France; Moises Diago, General Universitario, Valencia, Spain; Amy Lin, F. Hoffman-La Roche Ltd, Nutley, NJ; Edward Connell, F Hoffman-La Roch Ltd, Nutley, NJ
Eradication of HCV with interferon-based therapies is associated with improvements in health-related quality of life, improvement in liver histology, and prevention of liver-related death. Although the benefits of viral eradication have been established, knowledge of the overall durability of SVR in treated patients is limited. We aimed to quantify the long-term durability of SVR achieved with Pegasys monotherapy or Pegasys/Copegus combination therapy in patients with chronic hepatitis C who participated in pivotal trials up to 5 years previously.
Participants in phase II/III randomized, multinational trials of Pegasys alone or combined with Copegus were eligible for this long-term follow-up trial. SVR was defined as undetectable serum HCV RNA (40 have been followed for >=5 years. 174 received Pegasys monotherapy and 671 received various Pegasys/Copegus combination regimens.
To date >99% of patients have remained HCV RNA negative during long-term follow-up. 7 patients (<1%) have become HCV RNA positive, although whether these cases represent re-infection or true 'relapse' is at present unknown.
All HCV monoinfected patients who were treated for 48 weeks with Pegasys/Copegus 1000/1200 mg/d, and all 'normal' ALT and HIV-HCV co-infected patients have remained HCV RNA negative during follow-up.
The authors conclude that SVRs achieved with Pegasys, alone or in combination with Copegus, are durable for up to 5 years after completion of therapy in a broad spectrum of patients with chronic hepatitis C, including those with persistently 'normal' ALT levels and HIV-HCV co-infection.
|
|
| |
| |
 |
|
| |
| |
|
| |
|
 |
 |
|
|