 |
 |
 |
| |
SCH-D, CCR5 Receptor Inhibitor, new HIV Drug in New Class of Drugs:
|
| |
| |
antiviral effect, safety, pharmacokinetics, susceptibility
Reported by Jules Levin
For the first time the results of a phase I clinical study for a second generation CCR5 receptor antagonist, SCH-D, was presented at the Retrovirus Conference in San Francisco on Feb 12, 2004.
Mark Laughlin reported the study results for Schering Plough, which has had an angoing development program for CCR5 entry inhibitors. Two clinical candidates, SCH-C and SCH-D, have emerged from this program. SCH-C and SCH-D efficiently block cell entry of a wide range of primary HIV-1 isolates that use the CCR5 receptor for infection (R5 viruses). These compounds do not inhibit HIV isolates that gain entry into cells via the CXCR4 receptor (X4 viruses). Previously reported were Phase I results for SCH-C in HIV-infected patients. Due to toxicity observed SCH-C was discontinued to develop SCH-D, which has shown greater in vitro potency than SCH-C and has favorable pharmacokinetic and metabolism profiles in several animal species.
Laughlin reported a comparison of pharmacokinetics between SCH-C and SCH-D:
| SCH-C | SCH-D |
| Rat | Monkey | Rat | Monkey |
| 10mg/kg | 2mg/kg | 10mg/kg | 2mg/kg |
| Absortion | 57% | 85% | 100% | 95% |
| Bioavailability | 63% | 68% | 100% | 89% |
| Half Life | 5.4 hrs | 5 hrs | 7.9 hrs | 3.4 hrs |
| Cmax (ug/mL) | 1.5 | 0.30 | 2.3 | 0.67 |
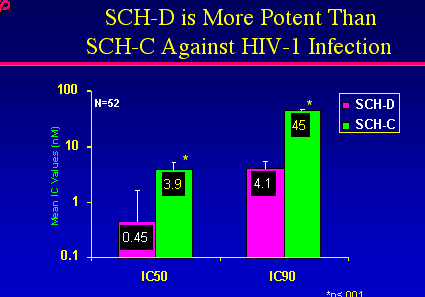
Regarding potency, Laughlin reported less drug of SCH-D is needed to provide similar viral suppression, therefore it appears more potent in in vitro studies::
|
|
| |
| |
 |
|
| |
| |
SCH-D MONOTHERAPY
Forty eight patients chronically infected with HIV were enrolled into a sequential rising-dose study of SCH-D 10 mg, 25 mg and 50 mg BID for 14 days. Within each of the three cohorts (N = 16), 12 patients received SCH-D and four patients received placebo in a randomized, blinded design. Patients were required to have been on no antiretroviral treatment for at least eight weeks prior to enrollment and to have a CD4+ cell count greater than 200/mm3.
Patients in this study had CD4 >250, HIV viral load 5,000-200,000 copies/ml; no antiretroviral therapy within 8 weeks; started study in-patient:
--cohort 1: 10 mg bid (twice daily) x 14 days, 12 active/4 placebo
--cohort 2: 25 mg bid x 14 days, 12 active/4 placebo
--cohort 3: 50 mg bid x 14 days, 12 active/4 placebo
STUDY ENDPOINTS
--viral kinetics, onset & rebound
--safety, tolerability, & PK
PATIENT DEMOGRAPHICS
Age was 38-40 yrs across the 3 study groups; Cd4 counts 369-472; viral load 36,000 copies/ml in 10 mg dose group, 81,000 c/ml in placebo, 43,000 c/ml in 25 mg, and 105,000 copies/ml in 50mg group; gender: 1-3 females and 8-12 men across the 3 groups.
SCH-D Phase I Study Results
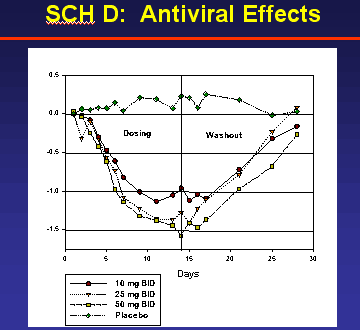
In the study, SCH-D was safe and well tolerated at all dose levels in these patients. The study did not identify any drug-specific toxicity in humans. The study showed a dose-related decrease in viral load, with mean Log10 reductions from baseline of 1.08, -1.56 and -1.62 in the 10, 25 and 50 mg BID dose groups, respectively. The percentage of patients with a >1.0 Log10 reduction in viral load was 55%, 69% and 81% in the 10, 25 and 50 mg BID dose groups, respectively. The percentage of patients with a >1.5 Log10 reduction in viral load was 27%, 46% and 45, respectively.
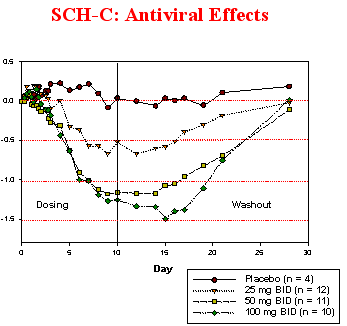
As you can see by comparing the antiviral effects of SCH-D and SCH-C from early pilot studies SCH-D appears to be more potent when comparing viral load reductions of the same 50 mg dose.
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
SAFETY UPDATE
Cohort 1: no serious adverse events
Cohort 2: 3 serious adverse events
--1 fever, reported + BC for mycobacterium, subsequently no growth
--1 2∞ syphilis, VDRL + 1;2 and treponema sp + 1"640 at baseline
--1 CVA (during screening period.
Cohort 3: no serious adverse events
Laughlin summarized that all doses were safe and well tolerated. That there was a clear in vitro and in vivo potency correlation; continued vigilance in monitoring for the presence of X4 is required; CCR5 receptor remains an appropriate target for antiretroviral therapy. Phase II studies are expected to start in first half of 2004.
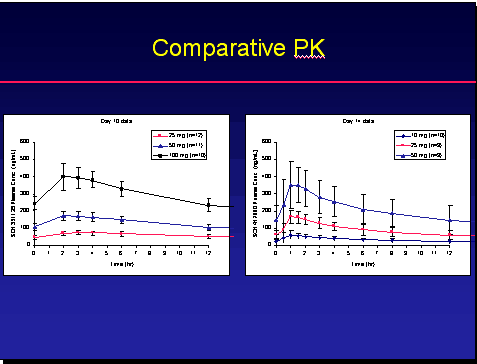
PHARMACOKINETICS
As you can see from the chart 50 mg of SCH-D showed superior blood levels and PK than SCH-C. The blue lines are the 50 mg dose.
|
|
| |
| |
 |
|
| |
| |
SUSCEPTIBILITY
--susceptibility to SCH-D determined at baseline and days 7, 14 and 28: no change from baseline in IC50 to SCH-D during dosing or follow-up
--one subject with mixed R5:X4 at baseline in high dose group Æ about 0.5 log10 reduction during dosing
--one subject (>1.5 log10 reduction during dosing) with transient detection of X4 following cessation of dosing
|
| |
|
 |
 |
|
|