 |
 |
 |
| |
Reyataz Expanded Access: lipids, viral response, safety
|
| |
| |
"ATAZANAVIR PLASMA LEVELS ARE ASSOCIATED WITH EFFICACY AND SAFETY IN PROTEASE INHIBITOR-EXPERIENCED HIV-INFECTED PATIENTS"
Ana Barrios, Ana Lucía Rendón*, Pilar Ríos**, Luz Martín-Carbonero, Daniel González de Requena*, Oscar Gallego**, Luisa Valer**, Ivana Maida, Teresa García-Benayas, Inmaculada Jiménez-Nácher*, Juan González-Lahoz and Vincent Soriano
Service of Infectious Diseases, *Pharmacokinetic Unit, and **Laboratory of Molecular Biology. Hospital Carlos III, Madrid, Spain
Here is text of poster.
ABSTRACT
Background: Atazanavir (ATV) is a well tolerated protease inhibitor (PI), but concerns have risen about its potency. ATV plasma levels might be useful to predict its efficacy and toxicity.
Methods: The clinical performance of ATV was examined in PI-experienced patients included in the expanded access program in a reference center in Madrid. ATV plasma levels (C12h; estimated Cmin) were measured (HPLC-UV) at week 12. The genotypic inhibitory quotient (GIQ) was defined as C12h/number of protease resistance mutations (PRM). All individuals received ATV 400 mg once a day with two additional drugs.
Results: Data from 92 and 52 patients followed for 12 and 24 weeks, respectively, were analysed. Median age, 42 years; male, 68%. Time on prior HAART, 72 months.
In patients with baseline VL >50 cop/ml (65%), the median plasma HIV-RNA was 3.9 log10 (10,000 copies/ml). Median CD4 count, 395 cells/ul. HCV coinfection, 45%. ATV was prescribed as rescue (45%), simplification (11%), or to ameliorate dyslipemia (24%) or other toxicities (16%). Concomitant tenofovir (TDF), 78%. None received ritonavir boosting.
At 24 weeks, virologic response (VR) (≥1log drop in HIV-RNA or to <50 cop/ml) was recorded in 64% (OT-analysis) or 57% (ITT). In patients with baseline detectable VL, median HIV-RNA drop was 0.7 log. Median CD4 gain was 38 cells/ul. Median ATV Cmin was 0.12 Ìg/ml (IQR, 0.05-0.22 Ìg/ml).
Immunologic and virologic responses did not correlate with ATV Cmin. Neither the efficacy of ATV nor Cmin significantly varied according to TDF use.
The number of PRM (IAS-USA list) tended to be associated with the VR: median of 5 PRM in failing patients vs 1 in responders (p=0.07). In subjectswith baseline VL>50 cop/ml, a higher VL drop was associated with a higher GIQ (p=0.02; ‚ -0.6; 95%CI –0.9,-0.2). At 24 weeks, only 4 patients (4%) discontinued treatment due to ATV-related toxicity (1 hyperbilirubinemia).
Although bilirubin increased overall in up to 66% of subjects, grade 4 only developed in 10%. Increases in bilirubin at week 12 were associated with higher ATV plasma levels (p=0.01; ‚ 0.4; 95%CI 0.3-1.1). No significant liver enzyme elevations were observed. There were no significant differences in bilirubin nor transaminases according to HCV status. Hypertriglyceridemiaand hypercholesterolemia significantly decreased.
Conclusions: ATV-based regimens provide a significant virologic response in PI-experienced patients, particularly in subjects lacking protease resistance mutations. The GIQ predicts accurately the virologic response to ATV. Hyperbilirubinemia is associated with ATV plasma levels.
Author's Summary & Conclusions: Atazanavir (ATV) efficacy and safety were evaluated in 92 PI-experienced patients included in the expanded access program in Madrid. At week 24, virologic response was recorded in 64% (OT) and 57% (ITT) of patients. Virologic response did not correlate with ATV Cmin. The number of protease resistance mutations (PRM) was lower in responder subjects than in non responders. In patients receiving ATV as rescue therapy component, a higher HIV-RNA reduction was associated with a higher GIQ.
Hyperbilirubinemia was asociated with higher ATV plasma levels. Finally, lipid profile clearly improves from baseline and the rates of hypertriglyceridemia and hypercholesterolemia declined significantly.
Unboosted ATV-based regimens are safe and provide significant virological response in PI-experienced patients, mainly among those with a low number of protease resistance mutations.
The GIQ predicts accurately the viral load reduction in patients receiving ATV containing regimens as rescue therapy.
Switch to ATV in heavily pretreated patients improves lipid profile significantly.
INTRODUCTION
Atazanavir, the latest approved HIV protease inhibitor (PI), is well tolerated and administered once a day, but concerns have risen about its potency and genetic barrier to resistance. These aspects may be particularly relevant when ATV is planned to be used in PI-experienced patients, in whom PRM may have been selected under previous treatment regimens. In this setting, ATV plasma levels might be useful to predict its efficacy and toxicity.
METHODS
All patients included in the ATV expanded access program in a large reference HIV/AIDS hospital in Madrid, and having had prior PI exposure were examined. All individuals received ATV 400 mg once a day with two additional drugs.
The predictive value of drug resistance genotypes, ATV plasma through levels and genotypic inhibitory quotient (GIQ) on the virological response at week 24 was assessed.
Drug resistance genotypes were determined retrospectively in plasma specimens collected before initiating ATV-based regimens. Resistance mutations at the reverse transcriptase and protease genes were identified following the list recorded by the IAS-USA Panel (CID 2003;37:113-28).
ATV plasma through levels were measured (HPLC-UV) at week 12, when the steady state already had been obtained. Blood was drawn in fasting conditions 12 hours after taking the last dose of ATV. The Cmin was estimated accordingly. GIQ was defined as Cmin/PRM. CD4+ cell count, plasma HIVRNA, bilirubin, transaminase and lipid values were evaluated at12 and 24 weeks.
RESULTS
Data from 92 and 52 patients followed for 12 and 24 weeks, respectively, were analyzed. Their median age was 42 years-old. Up to 68% were male. Their median time on prior HAART was 72 months. The median CD4 count was 395 cells/µl.
In patients with baseline plasma viral load > 50 cop/ml (65%), the median plasma HIV-RNA was 3.9 log10 (10,000 copies/ml). HCV coinfection was present in 45%.
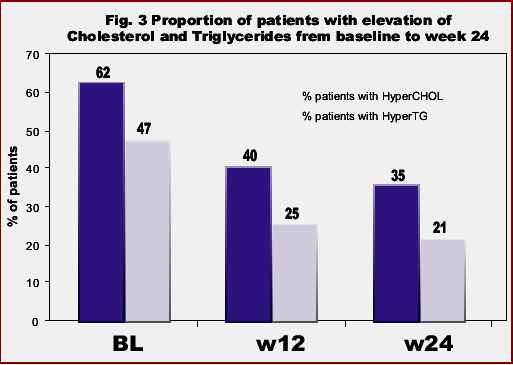
Accordingly to baseline plasma lipid values, 62% of subjects had hypercholesterolemia and 47% hypertriglyceridemia.
ATV was prescribed as part of a rescue intervention (45%), simplification strategy (11%), or in attempt to ameliorate dyslipemia (23%) or other toxicities (16%). Tenofovir (TDF) was concomitantly used with ATV in up to 78% of patients. None of them received ritonavir boosting.
At week 24, virologic response (VR) (≥ 1 log drop in plasma HIV-RNA or to < 50 cop/ml) was recorded in 64% of patients on therapy (OT) and 57% in the intent-to-treat analysis. In patients with baseline detectable viral load, VR was achieved in 52% of subjects and the median HIV-RNA drop was 0.7 logs (IQR, 0.2-1.8). The median CD4 gain was of +34 cells/µl (IQR, -10 to +78).
In this scenary, using unboosted ATV and with most of patients taking TDF concomitantly, the median ATV Cmin was 0.12 µg/ml (IQR, 0.05-0.22 µg/ml), which is clearly above the IC90 for ATV (0.014 µg/ml) testing wild type viruses (AAC 2000;24:417-24).
Neither virological nor immunological responses correlated significantly with ATV Cmin. There were no significant differences between median ATV levels in patients taking or not TDF concomitantly (0.11 µg/ml versus 0.12 µg/ml; p=0.9). Neither virological nor immunological responses significantly differed according to receiving or not concomitant TDF.
At 6 months, median cell count increase was of +34 cells/µl (IQR, -19 to +119) with TDF, and +75 cells/µl (IQR, 5-201) without TDF (p=0.4). VR (OT) was recorded in 61% (19/31) of subjects taking TDF versus 75% (6/8) without TDF (p=0.7).
The median number of PRM in the study population was 2 (IQR, 1-5.5), and tended to be lower in patients showing VR than in nonresponders(1 vs. 5 ; p=0.07). In subjects with baseline viral load > 50 cop/ml, a higher GIQ was associated with a higher viral load drop (p=0.02; ‚ -5.4; 95%CI -10 to -1).
At 24 weeks, only 4 patients (4%) discontinued treatment due to a potential ATV-related toxicity (hyperbilirubinemia in one). Although bilirubin elevations were recorded in up to 66% of subjects, grade 4 only developed in 10% and led to treatment discontinuation in only one patient. The extent of bilirubin elevations were associated with higher ATV plasma levels (p=0.06; ‚ 3.2; 95%CI -0.1 to 6.5). No significant liver enzyme elevations were observed. There were no significant differences in bilirubin nor transaminase increases according to HCV status.
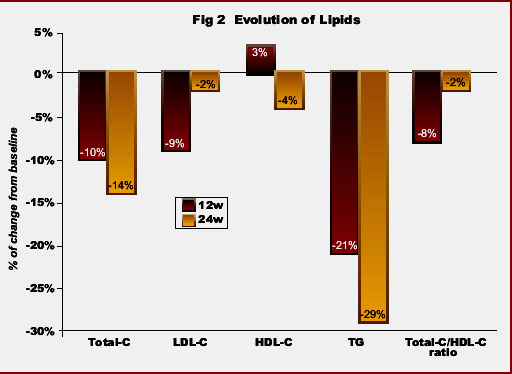
In the overall population, median triglycerides and total cholesterol levels, as well as the ratio total-/HDL-cholesterol significantly declined under ATV therapy. At week 24, values of triglycerides decreased -29%, total cholesterol -14%, LDL-cholesterol -2%, the ratio total-/HDL-cholesterol -2% and HDL-cholesterol -4% (Fig 2). Moreover, the proportion of patients with hypertriglyceridemia and hypercholesterolemia significantly declined in respect to baseline: from 47% to 21% and from 62% to 35% at 6 months, respectively (Fig 3).
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
|
| |
|
 |
 |
|
|