 |
 |
 |
| |
Ribavirin-NRTIs Interaction Study
|
| |
| |
Reported by Jules Levin
"Effect of Ribavirin on Intracellular and Plasma Pharmacokinetics of Nucleoside Reverse Transcriptase Inhibitors in Patients With HCV/HIV Co-infection: Final Results of a Randomized Clinical Study"
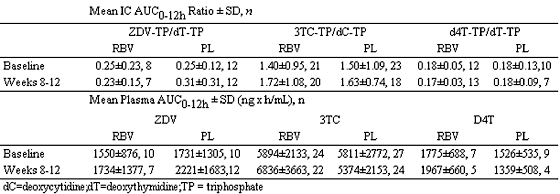
Researchers from Roche reported at Retrovirus on a study looking at 800 mg of ribavirin and nukes for potential drug-drug interactions. They examined patients in the APRICOT Study which was a trial of coinfected patients who received Pegasys plus Copegus (ribavirin), interferon plus ribavirin, or Pegasys monotherapy. The researchers found that RBV (800 mg/day) does not alter the intracellular phosphorylation or plasma pharmacokinetics of ZDV, 3TC, or d4T in HCV/HIV-co-infected patients when assessed after 8 to 12 weeks of combination therapy.
In a separate report at the conference, David Back and colleagues presented results from a study examining ribavirin 1000/1200 mg and NRTI interactions. He found that although AZT levels were increased when combined with RBV he does not think this will change AZT efficacy, but might increase AZT side effects/toxicity reducing hemoglobin counts while on ribavirin therapy. D4T and 3TC did not appear to be affected.
Whether ribavirin interferes with the pharmacokinetics of antiretroviral drugs is an important, but unanswered question relevant to the treatment of HCV in HIV-infected persons. Our objective was to examine the effect of RBV on intracellular phosphorylation of zidovudine (ZDV), lamivudine (3TC), and/or stavudine (d4T), and to determine whether plasma levels of RBV or these NRTIs are altered by co-administration.
This prospective nested pharmacokinetics study was incorporated into the design of the AIDS PEGASYS Ribavirin International Co-infection Trial (APRICOT), a randomized, controlled, multinational trial in 868 patients with HCV/HIV co-infection. Eligible HCV/HIV-co-infected patients were receiving a highly active antiretroviral therapy (HAART) regimen that included stable doses of ZDV plus 3TC, or d4T plus 3TC for at least 6 weeks prior to enrollment. Eligible patients were randomized to treatment with peginterferon-a-2a (40KD) (Pegasys) 180 ug/wk plus either oral RBV (COPEGUS) 800 mg/day or placebo; those randomized to interferon/RBV were excluded. Serial blood samples were collected predose (0) and 2, 4, 6, 8, and 12 hours after the morning dose of ZDV, 3TC, and/or d4T, at baseline (pretreatment) and after 8 to12 weeks of combination therapy. Concentrations of RBV, ZDV, 3TC, and d4T were determined in plasma by LC/MS/MS and the triphosphate anabolites of ZDV, 3TC, and d4T in peripheral blood mononuclear cells (PMBC) by template primer extension assays. Endogenous nucleotides (dTTP and dCTP) were also determined by primer extension assays. The area under the concentration-time curve (AUC 0-12h) was calculated and analyzed by ANCOVA.
Of 55 patients, 48 completed the study, with the following results:
|
|
| |
| |
 |
|
| |
| |
RIBAVIRIN 1000/1200mg -NRTIs
"Differential Effects of Combined Pegylated Interferon and Ribavirin Therapy on Intracellular Nucleotide Triphosphate Levels in HIV/HCV Co-infected Patients; a Potential Mechanism for Enhanced Toxicity"
David Back and colleagues from the University of Liverpool, UK reported these study results. He also participated in the Roche study reported above.
Combination treatment with pegylated interferon (peg-IFN) and ribavirin (RBV) delivers sustained virological response to HCV infection but is associated with significant toxicity. Anemia and neutropenia limit therapy or force reductions in RBV dose potentially compromising efficacy. Intracellular phosphorylation of RBV and the nucleoside analogues defines their efficacy and toxicity. ZDV, D4T, and RBV are competing substrates for a thymidine phosphorylation pathway raising concerns regarding adverse interactions and altered efficacy.
A pharmacokinetic investigation of the effect of combination peg-IFN/ RBV therapy (1000/1200 mg) on intracellular ZDV (n = 6), d4T (n = 5), and 3TC (n = 9) triphosphate levels in HIV suppressed, HCV-co-infected patients. Blood was reserved at 5 time points 0 to 8 hours, at baseline and on days 3 and 14. Intracellular triphosphates and endogenous nucleotide triphosphate levels in PBMC were determined using an enzymatic template primer extension assay.
In nongenotype 1 patients there was an 86% response (12 week). Hemoglobin decreased (9.6±1.4 vs13.9±0.7 g/L; p <0.001) and lactate dehydrogenase increased (860±250 vs 420±100U; p <0.001) in patients receiving ZDV compared with other nucleoside analogues. This was managed without RBV dose reduction. There was a 44% reduction in the ZDVTP/dTTP ratio (3.5±0.8 vs 2.18±0.2; p <0.02) from day 3 onwards. Interestingly this represented a 1.8-fold increase in absolute ZDVTP but a 2.95-fold increase in dTTP (for ZDV) levels. Both the d4TTP/dTTP ratio and dTTP (for d4T) concentrations were unchanged over the study period. The 3TCTP/dCTP ratio was increased 1.4-fold on day 3 reflecting an increase in levels of 3TCTP compared with baseline, this normalised by day 14.
Consistent with in vitro findings RBV decreased ZDVTP/dTTP phosphorylation. This was not associated with loss of ZDV efficacy. Reports suggest that RBV increases the endogenous dTTP pool providing negative feed back on thymidine kinase, reducing ZDV phosphorylation. However in this study absolute ZDVTP levels increased which may contribute to the increased toxicity observed in this group. In our study dTTP (for d4T) was unaltered failing to support a universal rise in dTTP levels as a result of RBV therapy. However, up-regulation of thymidine kinase by the combined presence of both RBV and ZDV but not d4T may explain both the increase in ZDVTP, increased dTTP (for ZDV) levels and lack of effect on dTTP for d4T.
|
| |
|
 |
 |
|
|