| |
Treatments for Hepatitis B
|
| |
| |
Clinical Infectious Diseases Nov 1, 2004;39:1353-1362
David M. Asmuth, Hien H. Nguyen, Gregory P. Melcher, Stuart H. Cohen, and Richard B. Pollard
Division of Infectious Diseases, University of California Davis Medical Center, Sacramento
SUMMARY: New optimism surrounds treatments for chronic hepatitis B (CHB). Interferon-α, lamivudine, and adefovir dipivoxil are currently approved by the United States Food and Drug Administration for the treatment of CHB. All 3 treatments possess unique characteristics with respect to their side effect profiles, potencies, and treatment niches within the spectrum of CHB. New agents, which are in various stages of clinical development, represent potential improvements within existing, as well as novel, classes of antiviral therapy, and they offer significant promise of a cure for the many patients with chronic and progressive hepatitis B. However, there remain many challenges in understanding the implications of drug resistance, the role of combination therapy, and how to define the response to therapy within subsets of patients with hepatitis B.
TEXT OF ARTICLE
Hepatitis B virus (HBV) is a hepadnavirus that is noncytopathic and causes significant morbidity and mortality worldwide. It has a partially double-stranded, 3.2-kb, open circular DNA genomic structure. At present, an estimated 370 million people worldwide (6% of the world's population) have chronic hepatitis B (CHB). Although the United States has witnessed a >60% decrease in cases of acute hepatitis B during the past decade, the Centers for Disease Control and Prevention (Atlanta, GA) estimated that there were >1.25 million Americans with CHB in 2003. CHB remains one of the most common causes of liver disease in the United States, leading to cirrhosis, liver failure, and hepatocellular carcinoma (HCC).
Acute hepatitis B is associated with a wide spectrum of clinical outcomes, from spontaneous resolution to end-stage liver failure and/or HCC. The multiple host and viral factors that influence the outcome observed include the age, sex, and ethnicity of the patient, as well as the immune status of the patient and external or environmental factors (e.g., superinfection with hepatitis D virus or HIV, in addition to exposure to such toxins as alcohol). Viral factors include the HBV genotype, the emergence of resistance or mutants that escape immune surveillance, and the presence of mutations in the precore gene (i.e., the hepatitis B e antigen [HBeAg]).
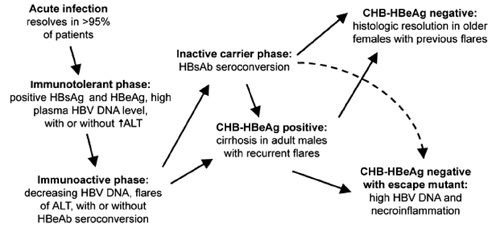
Natural history of hepatitis B virus (HBV) infection. ALT, alanine aminotransferase; CHB, chronic hepatitis B; HBcAg, hepatitis B core antigen; HBeAg, hepatitis B e antigen; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; ↑, elevated.
|
|
| |
| |
 |
|
| |
| |
The vast majority of patients with acute hepatitis B clear HBV and develop what is believed to be lifelong immunity to hepatitis B. An immunotolerant phase follows the acute phase of infection and is characterized by high levels of viral replication but no significant hepatic injury. Children—in particular, those with infection resulting from vertical transmission—have a prolonged immunotolerant phase.
The immunoactive phase, as its name implies, is characterized by the development of potentially incomplete immune control of viral replication. Decreasing plasma HBV DNA levels associated with flares of hepatic injury (which, in general, are defined by at least a 2-fold increase in the alanine aminotransferase [ALT] level, so that the ALT level is >10 times the upper limit of the range considered to be normal) can lead to either (1) complete resolution of the infection, with seroconversion to both hepatitis B surface antigen (HBsAg) and HbeAg; (2) an inactive carrier state with low levels of circulating viral surface antigen and minimal-to-absent hepatic injury; or (3) chronic active hepatitis B with ongoing hepatic injury and circulating HBeAg. Although a spontaneous hepatic flare can precede clearance of the virus, flares that occur during therapy can also signal drug resistance with increased viral replication. Although worsening hepatic insufficiency can be temporarily managed medically, uncompensated cirrhosis, end-stage liver disease, and development of HCC are associated with a very poor prognosis that, for a minority of patients, can only be salvaged by orthotopic liver transplantation. Alternatively, the immunoactive phase can spontaneously resolve with seroconversion to HBsAg and HBeAg.
Finally, the immunoactive phase of hepatitis B can evolve to include further immunologic escape with the development of a mutation in immunodominant epitopes, such as HBeAg leading to a "false-positive" clearance of HBeAg with or without seroconversion to HBeAg. HBeAg-negative individuals with CHB who have high viral loads often have significant hepatic injury, attendant increased morbidity and mortality, and reduced rates of response to current therapies. Certain genotypes (genotypes B, C, and D, but not genotype A) are characterized by a precore mutation (G1896A) that blocks production of HBeAg. Thus, regions where these genotypes predominate (i.e., southern Europe and Asia) report a higher frequency of CHB among HBeAg-negative individuals.
This labyrinth of potential pathways for the natural course of CHB has implications for the practicing clinician and for the design and interpretation of clinical trials. Frequently, before initiation of therapy, a 6-month observation period during which viral loads and transaminase levels are monitored is needed to clarify this issue. This observation period will allow the clinician to better pinpoint where a patient's condition fits into the continuum of disease progression and to recognize spontaneous viral clearance that can occur at any point. A clinical trial in which high response rates occur among the control group may signal a study population with heterogeneity in this regard that may influence the conclusions drawn from the trial. These considerations highlight both the role of liver biopsy before initiation of treatment and the need for surrogate markers to better characterize which patients are most in need and are most likely to benefit from antiviral therapy.
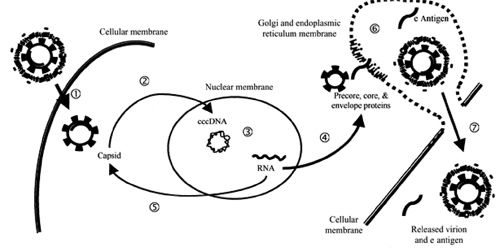
A brief overview of the life cycle of HBV will provide a framework for comparing classes of agents as well as a rationale for the use of combination therapy The virion traverses the cellular membrane, and, after disassembly, viral plus-strand DNA synthesis is completed by ligation of the open DNA strand, which leads to the formation of covalently closed circular DNA (cccDNA). This template for viral production serves as a reservoir for subsequent recurrence of previously cleared or treated infection. The 4 viral RNA transcripts produced from this template are transported into the cytoplasm, where translation of viral proteins and assembly of the viral capsid occur. Capsid assembly involves reverse transcription of the viral RNA to the viral DNA and is the target of therapeutic intervention with reverse-transcriptase inhibitors at the present time. Subsequent migration of the capsid and the remaining viral proteins into the Golgi apparatus and the endoplasmic reticulum leads to final virion assembly and secretion of mature viral particles. This secretory pathway characterizes the noncytopathic nature of HBV infection. Alternatively, after reverse transcription and completion of viral DNA replication, HBV again traverses the nuclear membrane to amplify the cccDNA pool. This pool will persist with a variable half-life, and it likely is transferred to daughter hepatocytes after mitosis. Thus, the hepatocyte life span and viral kinetics will influence considerations regarding duration of therapy. The goal of therapy may include clearance of both plasma viral DNA and the reservoir of cccDNA present in the nuclei, as well as seroconversion to HBsAg and/or HBeAg.
Replication cycle of the hepatitis B virus: step 1, viral entry; step 2, disassembly, completion of DNA plus-strand, and transnuclear membrane migration where covalently closed circular DNA (cccDNA) is formed; step 3, mRNA transcription from cccDNA; step 4, viral protein translation and capsid assembly in the cytoplasm; step 5, viral capsid and pregenomic RNA replenish the pool of cccDNA; step 6, assembly of viral particles in the Golgi apparatus and the endoplasmic reticulum; and step 7, noncytopathic release of virions.
|
|
| |
| |
 |
|
| |
| |
CURRENTLY AVAILABLE THERAPIES
The 3 approved therapies for CHB are IFN-α, lamivudine, and adefovir dipivoxil. Clinical trials examining these therapies have spanned nearly 3 decades, making comparison of the primary data difficult. For example, a common outcome that was measured across clinical trials was virologic response, which was defined as an undetectable viral load. However, the sensitivity of the viral assays has varied. Early trials of lamivudine used the Abbott hybridization assay, which has a lower limit of detection of 500,000 copies/mL. Clinical trials of adefovir dipivoxil used a more sensitive PCR assay, which has a lower limit of detection of 400 copies/mL. Early trials of IFN-α used unapproved viral assays, although more recent data on the use of pegylated IFN seem to corroborate the viral suppression response rates observed in early trials.
The goal of clinical trials of therapy for CHB has been clearance of and seroconversion to HBeAg. Although other end points, including seroconversion to HBsAg, seem to represent complete viral clearance, the importance of seroconversion to HBeAg was emphasized by recent data that suggested a 60-fold increased risk of HCC developing among HBeAg-positive individuals. Subsequently, in the present article, the discussion of seroconversion in clinical trials will focus on HBeAg, unless specifically noted otherwise.
IFN-&alpha
"Interferon," a term that was derived nearly a half century ago to describe a compound that causes viral interference, was the first therapy approved for the treatment of HBV infection. As a host cytokine produced in response to a viral infection, IFN-α has antiviral and immunomodulatory effects. Its antiviral activity is mediated by the induction of intracellular signals that are initiated after binding to the IFN-α cell membrane receptor. IFN-α exhibits potent antiviral activity by inhibiting viral penetration, translation, transcription, protein processing, maturation, and release [potentially, all steps]. In addition, the immunomodulatory effects of IFN-α are, in part, mediated by increasing the expression of major histocompatibility complex antigens, enhancing the phagocytic activity of macrophages, and augmenting the proliferation and survival of cytotoxic T cells. The latter is specifically believed to be an important mechanism of action of IFN-α when used as therapy for HBV infection.
Since the use of IFN-α for the treatment of CHB was first reported in 1976, IFN-α, in its various forms, has undergone numerous clinical trials to clarify its efficacy. Because of heterogeneous IFN formulations, subject populations, and clinical trial designs, the role of IFN-α in the treatment of CHB was difficult to demonstrate. While noting the inconsistencies across studies, a recent meta-analysis demonstrated, at 6--12 months of follow-up, that subjects treated with IFN-α showed significant benefit in terms of loss of HBeAg (33% of subjects vs. 12% of subjects who received placebo) and undetectable HBV DNA levels (37% of subjects vs. 17% of subjects who received placebo).
Among HBeAg-positive patients, predictors of response to therapy include low plasma HBV DNA levels, low amounts of hepatitis B core antigen (HBcAg) in the liver, high ALT levels, a high histologic activity index score at biopsy, acquiring infection as an adult and/or a history of acute hepatitis, and non-Asian ethnic origin. The response rate in specific subgroups of HBV-infected patients (e.g., patients with HIV-HBV coinfection or patients with precore mutants that fail to express HBeAg) is significantly lower. For chronically infected HBeAg-negative patients, low plasma HBV DNA levels predict a response to longer courses of therapy.
The addition of a pegylated moiety on IFN-α has significantly improved the pharmacokinetic and pharmacodynamic profile of IFN-α in the treatment of hepatitis C virus infection. The anticipated improved rates of response to IFN-α therapy among individuals with CHB have been realized in recent phase 2 clinical trials—in particular, among HBeAg-positive individuals. A total of 194 subjects with previously untreated CHB were involved in a 24-week dose-escalation trial that compared 3 doses of pegylated IFN-α2a (90, 180, or 270 μg weekly; Pegasys [Roche]) with conventional IFN-α2a (4.5 million IU given thrice weekly); results of the trial showed seroconversion to hepatitis B e antibody (HBeAb)--positive status in 37%, 35%, and 29% of subjects, compared with 25% of subjects, respectively, after an additional 24 weeks of follow-up [20]. Only 2%--4% of subjects discontinued receiving medication because of safety reasons. An additional 48-week trial that involved the use of pegylated IFN-α2a in combination with lamivudine provided encouraging virologic and serologic results, but these results were not sustained among HBeAg-negative subjects during follow-up. Phase 3 studies involving the use of pegylated IFN-α2a (40 kDa), 180 μg/week, are currently being designed and will better delineate the role of this approach in the treatment of CHB.
The mechanisms for resistance of HBV to IFN-α therapy are uncertain. However, mutations in the viral capsid proteins appear to suppress induction of Mx protein, an important antiviral effector molecule in the IFN-sensitive pathway, as a possible explanation for the significant failure rate associated with IFN therapy.
The potential side effects of IFN-α therapy include constitutional and psychiatric symptoms, neurotoxicity, hematologic abnormalities, and flares of hepatitis. IFN-α typically causes flulike symptoms, including headache, fevers, chills, myalgias, and malaise. Although these symptoms tend to improve over time, mood abnormalities may persist, resulting in either the development or exacerbation of mood disorders, including severe depression. Neurotoxicities include somnolence, confusion, and seizures. Hematologic side effects that may require treatment interruption or dose reduction include granulocytopenia and thrombocytopenia. Transient flares of hepatitis frequently occur during treatment, resulting in increases in the ALT level to ⩾2 times the levels noted at baseline. These episodes are more likely to occur among responders (i.e., subjects with response to IFN-α therapy) 8--12 weeks after initiation of therapy. In general, treatment should be continued unless signs and symptoms of liver failure are observed. Because of the possibility of a hepatitis flare and liver failure occurring, IFN-α therapy should be avoided for patients with decompensated cirrhosis. In addition, IFN-α therapy may cause thyroid disease and may exacerbate or unmask autoimmune diseases.
In summary, the use of IFN-α therapy for patients with CHB results in sustained response rates in approximately one-third of patients when therapy is given for 16 weeks (to HBeAg-positive patients) or for 52--104 weeks (to HBeAg-negative patients). These virologic and biochemical surrogate markers correlate with the following end points of clinical disease: liver-associated complications, the need for liver transplantation, and, probably, HCC. Pegylated IFN-α formulations appear to be more conveniently dosed and may have higher associated response rates.
Lamivudine
Lamivudine, a nucleoside analogue, competitively inhibits reverse transcriptase by competing with deoxycytidine triphosphate for incorporation into DNA chains, resulting in chain termination. The median effective concentration (EC50) of lamivudine for the treatment of HBV infection is 0.01--5.6 mol/L.
Numerous limited and 2 large multicenter, randomized, placebo-controlled trials have established the use of lamivudine for HBeAg-positive and HBeAg-negative individuals with CHB. The rates of HBeAg seroconversion to HBeAb were 16%--17% for subjects who received a 12-month course of lamivudine, 100 mg/day, compared with 4%--6% for subjects who received placebo. Although the rates of seroconversion were low, in a comparison of subjects who received lamivudine with subjects who received placebo, lamivudine (1) effectively suppressed HBV DNA to undetectable levels in 44% of subjects (vs. 16% of subjects), (2) normalized ALT levels in 41% of subjects (vs. 7% of subjects), and (c) improved necroinflammatory scores by >2 points in 49%--56% of subjects (vs. 23%--25% of subjects). The strongest predictor of response was the ALT level noted at baseline before the initiation of therapy. Higher rates of response to lamivudine correlated with higher pretreatment levels of ALT. Seroconversion appears to be durable in 64%--77% of subjects at the end of 3 years of follow-up. The durability of response may be influenced by the continuation of lamivudine therapy after seroconversion. Recent data have suggested that continued treatment for 4--8 months after seroconversion leads to improved durability of response. This is likely related to the clearance of the HBV cccDNA reservoir, as discussed above.
Although the initial rates of response among HBeAg-negative subjects are high, long-term responses are infrequent. "Complete response," which was defined by loss of HBV DNA and ALT normalization, was 63% versus 6% for placebo recipients after 6 months of treatment with lamivudine, 100 mg/day. After 12 months of lamivudine therapy, the rate of complete response was 65%--74%. However, the durability of the responses, similar to the durability of the responses to IFN-α, was much lower. In fact, almost all patients experienced relapse after discontinuation of therapy, and most relapses occurred within the first 5 months after treatment was stopped.
In addition, HBV genotype and subtype appear to influence sustained response and resistance to lamivudine. Patients infected with HBV genotype B appear to have higher rates of response to lamivudine, and patients with HBV subtype ayw tend to have lower mean serum HBV DNA levels and less resistance to lamivudine, compared with patients with HBV subtype adw.
Prolonged lamivudine therapy increases the development of resistance to lamivudine. Mutations in the tyrosine-methionine-aspartate-aspartate (YMDD) locus of the HBV RNA--dependent DNA polymerase lead to resistance. Although prolonged therapy appears to increase the rate of HBeAg seroconversion from 17% to 27% among HBeAg-positive patients, resistance emerged in 38% of patients. In the long-term follow-up to the multicenter Asian trial that detected a rate of resistance of 14% at the end of 1 year of therapy, 38%, 49%, 66%, and 69% of subjects developed resistance to lamivudine at 2, 3, 4, and 5 years of therapy, respectively. A total of 19% of the subset of HBeAg-negative patients developed resistance to lamivudine after receiving therapy for 1 year, and resistance was seen among 44% of patients after 2 years of therapy.
Lamivudine is well tolerated, and the rate of associated adverse events was similar to that noted for placebo in randomized trials. The only major adverse event, which is not a drug toxicity per se, appears at the time of withdrawal of lamivudine and results in an increase in the HBV DNA level and the development of a hepatic flare. This syndrome has been best described in individuals with HIV-HBV coinfection, but it can also occur in monoinfected patients as well. Similarly, with the development of resistance and breakthrough viremia, this phenomenon can also occur.
In summary, because of its site of action in the life cycle of HBV, lamivudine does not affect cccDNA, and, consequently, it allows HBV to persist intracellularly until the host cell dies. Therefore, clearance of the infection relies on host defense mechanisms, as evidenced by the influence of baseline ALT levels on outcome. Prolonged therapy with lamivudine appears to improve rates of seroconversion and delay the progression of disease until resistance develops.
Adefovir dipivoxil (numerous adefovir reports of updated data can be found on NATAP website, use search engine)
Adefovir dipivoxil is the prodrug of adefovir, an acyclic phosphonated adenine nucleotide analogue. Intracellularly, adefovir dipivoxil undergoes 2 phosphorylation steps to compete with deoxyadenosine triphosphate to inhibit viral DNA polymerase and HBV reverse transcriptase, resulting in chain termination of DNA synthesis. Its EC50 for HBV is 0.2--2.5 mol/L. It may also have immunomodulatory effects by increasing natural killer cell activity and type I IFN induction.
Two large multicenter, randomized, double-blinded, placebo-controlled trials of adefovir dipivoxil have been reported, leading to approval of the drug by the US Food and Drug Administration in 2002. In a trial involving HBeAg-positive subjects with CHB, a comparison of subjects who received adefovir dipivoxil, 10 mg/day for 48 weeks, with subjects who received placebo revealed HBeAg seroconversion in 12% and 6% of subjects, histologic improvement in 53% and 25% of subjects, and undetectable HBV DNA levels in 21% and 0% of subjects, respectively. In a similarly designed trial involving HBeAg-negative patients with CHB, histologic improvement was noted in 64% of subjects who received adefovir dipivoxil versus 33% of subjects who received placebo, and undetectable serum HBV DNA levels were noted in 51% of subjects who received adefovir dipivoxil versus 0% of subjects who received placebo. In both trials, the long-term response rates while subjects were not receiving therapy were not reported; however, reemergence of viral replication would be expected because of incomplete suppression of the viral reservoir.
In contrast to findings regarding lamivudine therapy, mutations leading to clinical resistance were not detected during 2 years of adefovir dipivoxil therapy, although recent reports have identified mutations associated with virologic rebound after prolonged therapy. As the number and types of HBV therapies expand, adefovir dipivoxil may be useful in several areas. Its use for the treatment of lamivudine-resistant CHB has been promising. In clinical trials involving subjects with decompensated cirrhosis and in subjects after liver transplantation, adefovir dipivoxil was well tolerated, achieved significant reductions in HBV DNA levels, improved results of liver function tests, and improved Child-Pugh scores. Finally, HBV subtype adw appears to be intrinsically less responsive and more likely to develop resistance to lamivudine [61]. On the other hand, adefovir dipivoxil appears to be active for all HBV genotypes.
Adefovir dipivoxil, when administered in the 10-mg daily dose used for the treatment of HBV infection, appears to be relatively safe. In large trials, the adverse events associated with adefovir dipivoxil were similar to those noted in association with administration of placebo, except for the development of headache, asthenia, diarrhea, and abdominal pain. Events requiring discontinuation of therapy included increases in ALT or aspartate aminotransferase levels, weight loss, and rash. Although nephrotoxicity, which is seen in association with the higher doses of adefovir dipivoxil used for the treatment HIV infection, can still occur, it was not seen more frequently among subjects who received adefovir dipivoxil than among subjects who received placebo in pivotal clinical trials. When withdrawn, adefovir dipivoxil, like lamivudine, can also result in the development of a hepatic flare. In summary, although adefovir dipivoxil does not appear to be more potent than lamivudine, its advantages include treatment of infection due to the YMDD mutant, lamivudine-resistant HBV, and HBV genotypes that are less responsive to lamivudine, as well as a lower rate of resistance associated with long-term therapy.
NEW AGENTS UNDER DEVELOPMENT
A large number of agents that are the focus of preclinical and early clinical trials promise to enhance the effectiveness of the treatment options for CHB (table 1). When administered to treatment-naive subjects during phase 2 clinical trials, entecavir and telbivudine, 2 very potent and well-tolerated agents, have resulted in decreases of 5--6 log10 copies/mL in the plasma HBV DNA level after 48 weeks of therapy. Phase 3 trials of these and other promising agents are ongoing. Entecavir phase III studies are ongoing & study results are expected at AASLD in Oct 2004.
Therapies for hepatitis B virus (HBV) infection
| Agent | Class Structure | Targeted virus(es) | | Lamivudineb | Pyrimidine nucleoside | HBV and HIV ... | | Emtricitabine | Pyrimidine nucleoside | HBV and HIV | | Clevudine | Pyrimidine nucleoside | HBV and EBV | | Entecavir | Purine nucleoside | HBV | | Telbivudine | L-Pyrimidine nucleoside | HBV | | Valtorcitabine | L-Pyrimidine nucleoside | HBV | | Adefovir dipivoxilb | Purine nucleotide | HBV and HIV ... | | IFN-αb | Cytokine | HBV, HCV, and HHV-8 |
Potential immunomodulatory therapies for the treatment of CHB that are under development include therapeutic vaccination. Phase 1 studies involving a recent oral vaccination with HBV proteins demonstrated a reduction in the plasma viral load and an augmentation of the cell-mediated immune responses to HBV epitopes in a subset of subjects. These and other early investigations of immunomodulatory therapies provide evidence for new strategies for the control of CHB.
CHALLENGES FACING FUTURE TREATMENT EFFORTS
Although more-potent and more-specific antiviral treatments promise to improve the frequency and durability of responses to therapy among a wider spectrum of patients with CHB, many significant challenges remain. The emergence of resistance and management of resistance have implications broader than those discussed for each of the agents described in the present article. One important consideration comes from the realization that both the envelope and the polymerase gene map to overlapping regions in the HBV genome. Because of this structure, mutations in the polymerase gene can result in changes to the envelope as well. When immunodominant epitopes are involved, such polymerase gene mutations have been documented to lead to immunologic escape from vaccination and treatment failures.
From the practical standpoint of the resistance to and toxicity of different therapies, the duration of therapy for nonresponders (i.e., subjects with failure to have seroconversion to HBeAg) becomes problematic. Although lamivudine and adefovir dipivoxil have demonstrated the ability to normalize the ALT level and suppress viral replication, these drugs show a suboptimal rate of viral clearance, as measured by seroconversion to HBeAg. However, if these therapies are not continued indefinitely, hepatic flares can occur as a result of drug withdrawal. In addition, for HBeAg-negative patients, there is no biochemical marker of viral clearance, which makes it even more difficult to determine the duration of therapy. Although there presently are no firm recommendations regarding treatment duration for individuals who currently are receiving therapy, it appears reasonable to continue therapy indefinitely, until resistance develops (i.e., until return of the HBV DNA load to its level at baseline) or until clearance of the virus occurs for patients receiving lamivudine and adefovir dipivoxil. Nucleoside reverse-transcriptase inhibitors appear to be less toxic and easier to administer for a prolonged period, despite lower HBeAg seroconversion response rates, compared with IFN-α. Although most of the data regarding long-term IFN therapy are from early trials in which conventional IFN-α was used, the use of IFN-α is most practical when it is used in its pegylated formulation as salvage therapy.
As has been seen regarding therapy for infection with Mycobacterium tuberculosis or HIV, combination therapy is likely to play a significant role in the treatment of CHB by reducing the emergence of resistance. To date, there are limited available studies that use this approach, although many trials are presently in progress. The combination of lamivudine and IFN has been studied in a number of clinical trials, with none of the studies demonstrating a clear, sustained response to combination therapy that is greater than the response to monotherapy. As expected, for subjects for whom lamivudine therapy has failed, treatment with adefovir dipivoxil plus lamivudine has not demonstrated an advantage of combination therapy over adefovir dipivoxil therapy alone. Clinical trials that are currently in progress will exploit the strategy of targeting multiple steps within the life cycle of viral infection, to improve the durability of responses and to reduce the emergence of resistance.
Current recommendations regarding therapy focus on the presence or absence of HBeAg and HBV DNA levels and transaminase levels. Initiation of therapy is warranted for individuals with plasma HBV DNA levels of >105 copies/mL (100,000) (note from Jules Levin: some recommendations are to consider therapy at 10,000 copies/ml) and a significant increase in transaminase levels (to twice the upper limit of the range considered to be normal). Several consensus papers address current recommendations regarding initiation and duration of therapy, surrogate markers for monitoring progression of disease, and other important disease-management controversies. As more effective and better tolerated treatment modalities for the control of CHB become available, we can expect these management algorithms to evolve with an associated improvement in treatment response rates.
|
|
| |
| |
|
|
|