| |
Alcohol and Hepatitis C: Implications for Disease Progression and Treatment
|
| |
| |
Alexander Monto, MD
San Francisco Veterans Affairs Medical Center, 4150 Clement Street, San Francisco, CA 94121, USA.
Current Hepatitis Reports August, 2004, 3:105-111
"...Overall, data are emerging that light alcohol use leads to some increase in liver scarring in patients with hepatitis C, but that this effect is less than in heavy drinkers...
... Two recent studies suggest a similar role for light or moderate alcohol use on liver disease in patients with HCV...
... Heavy alcohol intake is clearly associated with cirrhosis, alone and particularly in combination with HCV. Cirrhosis has been shown to be the primary risk factor for hepatocellular carcinoma (HCC)...
... Studies addressing this question to date have generally not found an association between alcohol intake and steatosis (fat deposition in liver, which can accelerate disease & reduce response to PegIFN/RBV) in patients with chronic HCV, but this continues to be investigated...
... some clinical characteristics of the interaction between hepatitis C and alcohol, such as elevated HCV viral load in heavy drinkers, are well established...
... Five of the six studies found that alcohol had a negative relationship to the response to hepatitis C therapy... none of the studies reported patient compliance with therapy. As such, it remains unknown whether observed response differences seen in drinkers actually reflect biological differences, or whether the alcoholics were merely less compliant with therapy (note from Jules Levin: data suggests alcohol has detrimental biologic affect)."
ARTICLE TEXT
Patients with chronic hepatitis C are counseled by their physicians to abstain from alcohol intake. This recommendation is based on multiple epidemiologic studies showing that heavy alcohol intake correlates with worse hepatitis C virus-associated liver disease, as well as published international consensus guidelines. It is also logical, because alcohol intake is likely second only to hepatitis C as a cause of liver disease in the western world. Abstaining from alcohol removes a major hepatotoxin. When one evaluates critically what has been demonstrated about the relationship between hepatitis C and alcohol, however, the data remain limited. Heavy alcohol intake in association with chronic hepatitis C has been shown unequivocally, at a patient population level, to lead to worse liver disease. The role of light alcohol intake on disease remains controversial. Alcohol has been linked to the development of hepatocellular carcinoma, likely substantially through its role in the development of cirrhosis. Alcohol use also seems to lead to diminished response to interferon-based treatment of hepatitis C, although whether this is a direct effect is not known.
Hepatitis C virus (HCV) is the leading cause of cirrhosis and liver transplantation in the United States [1] and Europe [2]. After infection with HCV, approximately 85% of individuals fail to clear the virus, and develop a state of chronic, continuous viremia and some degree of hepatic injury. The outcome of hepatic injury, however, varies dramatically among individuals. Progression to cirrhosis differs based on the group examined, from 22% after 20 years of infection in studies from liver clinics, to 4% in blood donor series [3]. Disease progression clearly depends on a variety of factors.
Epidemiologic studies have shown that certain patient-related variables are associated with worse liver disease in the setting of chronic HCV. In general, these include older age at HCV acquisition, heavy alcohol consumption, and male gender [4]. Longer duration of HCV infection, older age, higher inflammation score on liver biopsy, and elevated serum liver enzymes have also been found to correlate with liver fibrosis in several studies [5, 6, 7, 8]. By contrast, method of HCV acquisition, HCV viral load, and HCV genotype have generally been found not to correlate with the severity of liver disease [4, 7, 8].
DEFINITION OF ALCOHOL INTAKE
The amount of alcohol a person drinks is inherently difficult to quantify. Seventy percent of members of western societies drink some alcohol regularly, and the amount tends to vary substantially over the course of an individual's life. Moreover, the amount of alcohol that causes harm has never been defined and likely differs from person to person. Large, population-based studies have found that more than one to two drinks per day in women [10] and two to three drinks per day in men [9, 10] correlate with the presence of alcohol-induced liver disease. Conversely, light alcohol intake has also been shown to lower all-cause mortality, suggesting that light intake benefits health [11]. Alcohol intake is generally quantified as grams of alcohol per day, with a typical "drink" containing 10 to 15 g of absolute alcohol. Some studies also use a threshold of alcohol intake with a time cut-off (eg, > 60 g/d for 5 years), whereas others estimate lifetime intake.
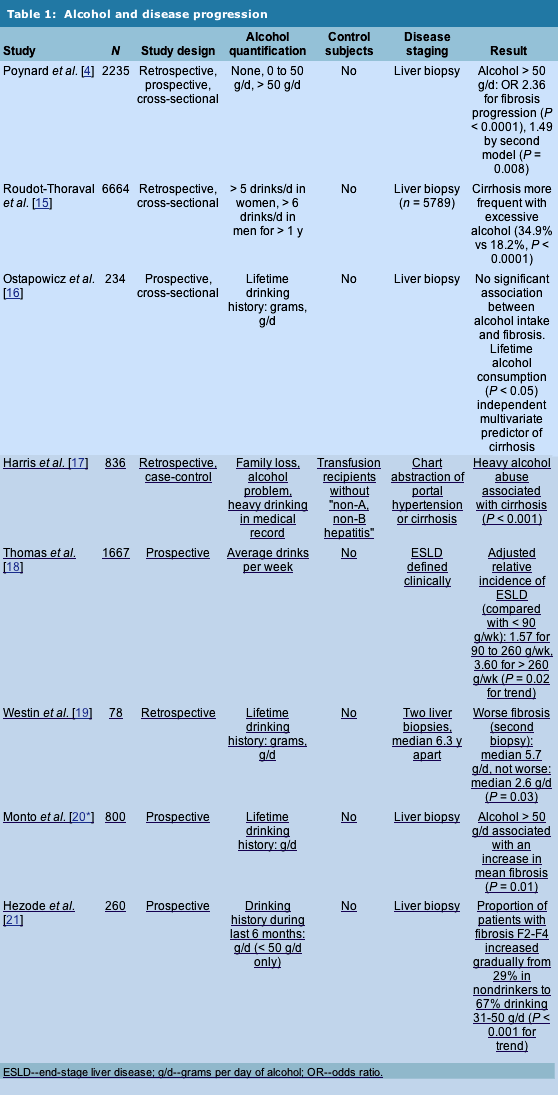
Role of Heavy Alcohol Intake in Disease Progression
Heavy alcohol consumption and hepatitis C have been known to interact with one another in causing liver disease since the discovery of the HCV in 1989 [12]. Numerous studies published in the early 1990s documented that in alcoholics, and particularly alcoholics with advanced liver disease, hepatitis C infection was common [13, 14]. Poynard et al. [4] were among the first to demonstrate that heavy alcohol intake (50 g/d or more in their study) contributes to fibrosis on liver biopsy independent of other predictors.
|
|
| |
| |
 |
|
| |
| |
Numerous other studies have confirmed that heavy alcohol intake contributes to disease progression in patients with chronic HCV. Table 1 lists some of these studies. Notable among them are the study by Roudot-Thoraval et al. [15], which evaluated over 6600 patients. Heavy alcohol intake in this study was associated with a significantly higher prevalence of cirrhosis than lower amounts of alcohol intake. Another important study was that of Ostapowicz et al. [16], in which patients with HCV who had undergone a liver biopsy had a detailed lifetime alcohol history obtained. Daily alcohol intake was not associated with fibrosis in multivariate analysis, but patients with cirrhosis had greater total lifetime alcohol consumption than patients who did not have cirrhosis. Limitations of studies published to date have included grouping subjects by fixed categories of alcohol intake [4], and using case-control methodology [17], with cases often having cirrhosis and control subjects having little liver disease.
Role of Light or Moderate Alcohol Intake in Disease Progression
Studies including light drinkers have had conflicting results. Ostapowicz et al. [16] found no significant relationship between light or moderate alcohol intake and fibrosis on liver biopsy. A second, prospective study, this one of inner-city injection drug users, found a statistically significant increase in the adjusted incidence of cirrhosis when greater than 90 g/wk and greater than 260 g/d were consumed [18]. The small, retrospective study found increased alcohol consumption (median 5.7 g/d) in the 44 patients whose fibrosis worsened over time compared with the 34 patients (median 2.6 g/d) whose fibrosis did not worsen [19].
Two recent studies suggest a similar role for light or moderate alcohol use on liver disease in patients with HCV. A group of 800 American patients who had lifetime alcohol use estimated at the time of liver biopsy were found to have an increase in mean fibrosis if they had drunk greater than 50 g/d of alcohol over their years of drinking, and lower amounts of alcohol were associated with fibrosis, but to a lesser extent [20*]. A French study of similar design found that quantity of alcohol drunk within 6 months prior to liver biopsy was also associated with fibrosis, and in a similar pattern lower amounts of alcohol exerted some effect, 31 to 50 g/d more [21]. Overall, data are emerging that light alcohol use leads to some increase in liver scarring in patients with hepatitis C, but that this effect is less than in heavy drinkers.
Alcohol and Hepatocellular Carcinoma
Heavy alcohol intake is clearly associated with cirrhosis, alone and particularly in combination with HCV. Cirrhosis has been shown to be the primary risk factor for hepatocellular carcinoma (HCC) [22]. Thus, alcohol is clearly associated with HCC through causing cirrhosis. It remains unclear, however, whether alcohol leads to liver cancer independent from its effect on cirrhosis.
A number of prospective studies have found alcohol to be an independent predictor of HCC [23, 24, 25]. Aizawa et al. [24] included histologic stage in their multivariate model, and found alcohol to be associated with HCC independent of histologic stage. However, stage had often been ascertained 5 to 15 years prior to HCC diagnosis. A large, case-control study of patients with HCV found that alcohol consumption greater than 60 g/d was associated with twice the risk of HCC as abstinence or light alcohol consumption [25]. A cross-sectional study from France, however, found that alcohol was not independently associated with HCC, but rather only cirrhosis was [15]. A recent, population-based study from Taiwan confirmed the importance of HCV as a risk factor for HCC, with presence of an anti-HCV giving a relative risk for cancer of 3.6. Alcohol intake added to this risk, with a further threefold increased incidence of HCC in patients with HCV who drank alcohol [26*].
Alcohol has also been linked to outcomes in patients diagnosed with HCC. In a Japanese cohort of 53 patients who underwent resection of HCC, patients who continued to consume greater than 80 g/d of alcohol had a significantly diminished median disease-free survival (12.6 months) compared with those drinking less than 80 g/d (25.4 months, P < 0.01) [27].
To summarize, alcohol intake has been linked to HCC development in the setting of chronic hepatitis C. The demonstration of this association at a population level, as well as between alcohol and HCC recurrence, suggests a direct link between alcohol and HCC independent of the effect of alcohol on fibrosis, but this has not yet been proven.
Possible Mechanisms of Interaction Between Alcohol and Hepatitis C
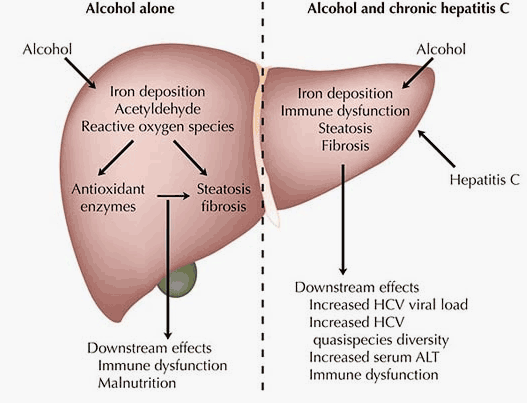
Alcohol outside the context of hepatitis C infection exerts specific pathologic effects on the liver [28, 29]. The degree to which these alcohol-specific effects cause disease in chronic HCV infection, compared to different effects caused by alcohol interacting with HCV, is unknown. Liver biopsies in patients with hepatitis C who drink alcohol are generally consistent with hepatitis C alone, with portal-based pathology, rather than alcoholic injury, which tends to be centrilobular [30, 31]. Such findings imply that hepatitis C is the primary disease process, and alcohol secondary. However, the histologic and pathologic effects of alcohol are likely transitory, and may resolve with periods of abstinence [32]. The principal pathologic effects of alcohol seem to occur when it is present at high levels, and thus are more episodic (ie, during periods of heavy drinking) than HCV, which is continuously present.
Alcohol exerts a variety of pathologic effects in the setting of chronic HCV. Many clinicians have noted that when patients with chronic HCV who regularly drink alcohol abstain for a period of time, they tend to lower their serum alanine aminotransferase levels [33]. Recent alcohol intake has been linked to an increase in HCV viral load [33, 34], and alcoholics have been found to have more HCV quasispecies diversity than patients with HCV who are not alcoholic [35].
|
|
| |
| |
 |
|
| |
| |
The underlying pathogenic mechanisms by which these clinical changes occur remain incompletely understood. Intrahepatic steatosis has been explored as possibly playing a pathogenic role, because it is characteristic of both alcoholic and HCV-associated liver injury independently [36]. Studies addressing this question to date have generally not found an association between alcohol intake and steatosis in patients with chronic HCV [31, 37], but this continues to be investigated [38]. Prior alcohol consumption has more recently been linked to both apoptosis [37] and to oxidative stress [38] in patients with hepatitis C, and these may turn out to be important mechanisms by which alcohol acts.
Immune dysfunction, documented with alcohol ingestion, has also been implicated in the interaction between alcohol and HCV [39, 40]. Substantiation of this has thus far been through two mouse models, rather than in humans. One study fed mice either an ethanol or an isocaloric diet and then immunized them against the HCV nonstructural protein NS5 [41*]. Ethanol-fed mice did not produce antibodies to NS5 and had suppressed interleukin-2 and interferon-g secretion compared with control subjects, and the antibody response recovered with removal of ethanol intake. The second model immunized mice against HCV core protein, and ethanol-fed mice had less HCV-associated T-helper and cytotoxic T-lymphocyte activity and reduced cytokine expression than control subjects [41*]. Recently, alcohol was also shown to lead to increased HCV replicon expression in liver cell lines in vitro, implying a further, direct role for alcohol in HCV replication, rather than necessarily an immune-mediated one [42].
In summary, although some clinical characteristics of the interaction between hepatitis C and alcohol, such as elevated HCV viral load in heavy drinkers, are well established, the mechanisms of the interaction remain unknown. Ultimately, a more detailed understanding of metabolic and immune changes brought about by alcohol in the setting of chronic HCV will be essential to defining these mechanisms.
Alcohol and Hepatitis C Treatment
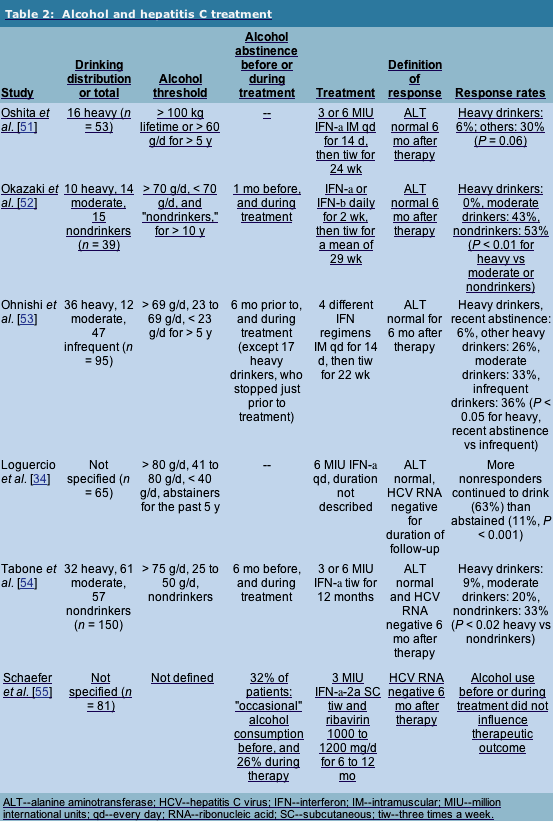
Active alcohol intake is considered a relative contraindication to interferon-based therapy [43, 44**]. This recommendation is based substantially on the documented noncompliance of alcoholics with different medical therapies [45], and the fact that the side effects of interferon therapy make it extremely difficult to comply with, even in patients without ongoing substance abuse [46]. Because abstinence prior to therapy is considered part of standard management, major treatment trials of anti-hepatitis C therapy excluded active drinkers [47, 48, 49, 50], and did not evaluate alcohol history as a predictor variable in treatment response. As such, the effects of alcohol on HCV treatment response are only gleaned from much smaller studies.
Nearly all studies of treatment responses in alcohol drinkers and nondrinkers have been in the setting of interferon monotherapy, and none have been blinded nor randomized (Table 2). The first three studies listed were performed in Japan. Sixteen of 53 patients in one study were defined as heavy drinkers, and only 6% responded biochemically to treatment, compared with a 30% response in nondrinkers [51]. A second study found that biochemical response segregated directly with alcohol intake, 53% in nondrinkers, 43% in those consuming less than 70 g/d of alcohol, and 0% in those consuming greater than 70 g/d [52]. A third study suggested that ongoing heavy alcohol use had further deleterious effects on treatment compared to past heavy alcohol use with several years of abstinence [53].
More recently, an Italian group published a study with design and results similar to the earlier Japanese studies [34]. Sixty-five Italian patients, the majority of whom were still drinking some alcohol, were treated with high-dose interferon. Sustained response to interferon was inversely proportional to the amount of alcohol consumed while on therapy. A second Italian study required abstinence from alcohol for 6 months prior to and during interferon monotherapy [54]. Sustained virologic response was significantly reduced in those with greater than 75 g/d alcohol consumption prior to abstinence (9%), compared with nondrinkers (33%).
Finally, in the only study treating patients with combination therapy, a German group treated 81 patients whose psychiatric, substance use, and alcohol use histories were characterized prior to, during, and after interferon and ribavirin therapy [55]. Twenty-one patients (26%) reported alcohol use while on therapy, and six of these required inpatient treatment for detoxification. No significant differences were found, however, in therapeutic outcome and alanine aminotransferase levels after treatment based on alcohol consumption before or during combination therapy.
Five of the six studies found that alcohol had a negative relationship to the response to hepatitis C therapy. This could lead to the hypothesis that the immune responses of heavy alcohol drinkers are different to those of nondrinkers, or that alcoholics are in some other way biologically different from other people. However, these were all small, nonrandomized, unblinded studies, so basing firm conclusions on them is difficult. Treatment regimens were largely interferon monotherapy, and responses were often biochemical rather than virologic. Moreover, none of the studies reported patient compliance with therapy. As such, it remains unknown whether observed response differences seen in drinkers actually reflect biological differences, or whether the alcoholics were merely less compliant with therapy.
CONCLUSIONS
Patients with chronic hepatitis C infection likely should not drink alcohol. Heavy alcohol use has been found in multiple studies to be associated with fibrosis progression and cirrhosis. Light and moderate alcohol consumption also seem to contribute to liver disease.
Alcohol intake in patients with HCV has also been linked to HCC. This may be principally through the association between alcohol and cirrhosis; studies have not demonstrated a link between alcohol and HCC independent from the association between alcohol and cirrhosis. Both past and current heavy alcohol use have also been associated with a decrease in patients' response to interferon-based therapy, but studies have largely been retrospective and small, and have not addressed whether patient compliance with therapy is also affected by alcohol. Finally, the mechanisms by which alcohol and hepatitis C interact remain investigational. It is likely that alcohol exerts a variety of pathologic effects in patients with HCV, including metabolic changes in the liver and alterations in the immune response to HCV, the latter having been suggested particularly by mouse models. Future studies with careful quantification and documentation of the timing of alcohol intake will shed additional light on all of these questions.
|
|
| |
| |
 |
|
| |
| |
References and Recommended Reading
Recently published papers of particular interest have been highlighted as:
* Of importance
** Of major importance
1.Wong JB: Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health 2000, 90:1562-1569.
2.Prieto M, et al.: Liver transplantation in hepatitis C: a Spanish multicenter experience. Eur J Gastroenterol Hepatol 1998, 10:771-776.
3.Freeman AJ, et al.: Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001, 34:809-816.
4.Poynard T: Natural history of liver fibrosis in patients with chronic hepatitis C. Lancet 1997, 349:825-832.
5.Yano M, et al.: The long-term pathological evolution of chronic hepatitis C. Hepatology 1996, 23:1334-1340.
6.Gordon SC, et al.: The significance of baseline alanine aminotransferase on pretreatment disease characteristics and response to antiviral therapy in chronic hepatitis C. Hepatology 2000, 32:400-404.
7.Danta M, et al.: Factors associated with severity of hepatic fibrosis in people with chronic hepatitis C infection. Med J Aust 2002, 177:240-245.
8.Poynard T, et al.: Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol 2001, 34:730-739.
9.Becker U, et al.: Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996, 23:1025-1029.
10.Bellentani S, et al.: Drinking habits as cofactors of risk for alcohol induced liver damage. Gut 1997, 41:845-850.
11.Gronbaek M, et al.: Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med 2000, 133:411-419.
12.Choo QL, et al.: Isolation of a cDNA clone derived from a blood borne non-A, non-B viral hepatitis genome. Science 1989, 244:359-362.
13.Mendenhall CL, et al.: Epidemiology of hepatitis C among veterans with alcoholic liver disease. Am J Gastroenterol 1993, 88:1016-1021.
14.Coelho-Little ME, et al.: Hepatitis C virus in alcoholic patients with and without clinically apparent liver disease. Alcohol Clin Exp Res 1995, 19:1173-1176.
15.Roudot-Thoraval F, et al.: Epidemiological factors affecting the severity of hepatitis C virus-related liver disease: a French survey of 6,664 patients. Hepatology 1997, 26:485-490.
16.Ostapowicz G, et al.: Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology 1998, 27:1730-1735.
17.Harris DR, et al.: The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med 2001, 134:120-124.
18.Thomas DL, et al.: The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000, 284:450-456.
19.Westin J, et al.: Moderate alcohol intake increases fibrosis progression in untreated patients with hepatitis C virus infection. J Viral Hepatitis 2002, 9:235-241.
20.* Monto A, et al.: Risks of a range of alcohol intake on hepatitis C-related fibrosis. Hepatology 2004, 39:826-834.
Large study that compared detailed alcohol history to liver histology at three centers, each with different levels of alcohol intake, allowing the effect of the full range of alcohol intake to be explored.
21.Hezode C, et al.: Impact of moderate alcohol consumption on histological activity and fibrosis in patients with chronic hepatitis C, and specific influence of steatosis: a prospective study. Aliment Pharmacol Ther 2003, 17:1031-1037.
22.Kew MC: Relationship between hepatocellular carcinoma and cirrhosis. Semin Liver Dis 1984, 4:136-146.
23.Kuwana K, et al.: Risk factors and the effect of interferon therapy in the development of hepatocellular carcinoma. J Gastroenterol Hepatol 1997, 12:149-155.
24.Aizawa Y, et al.: Analysis of factors affecting the appearance of hepatocellular carcinoma in patients with chronic hepatitis C. Cancer 2000, 89:53-59.
25.Donato F, et al.: Alcohol and hepatocellular carcinoma: the effect of lifetime alcohol intake and hepatitis virus infections in men and women. Am J Epidemiol 2002, 155:323-331.
26.* Sun CA, et al.: Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. Am J Epidemiol 2003, 157:674-682.
Population-based study of risk factors for liver cancer development, which included evaluation of alcohol intake along with viral hepatitis status.
27.Okada S, et al.: Effect of heavy alcohol intake on long-term results after curative resection of hepatitis C virus-related hepatocellular carcinoma. Jpn J Cancer Res 1996, 87:867-873.
28.Lieber CS: Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J Hepatol 2000, 32(1 suppl):113-128.
29.Walsh K: Alcoholic liver disease. Postgrad Med J 2000, 76:280-286.
30.Tamai T, et al.: Effects of alcohol consumption on histologic changes in chronic hepatitis C: a clinicopathologic study. Alcohol Clin Exp Res 2000, 24:106S-111S.
31.Monto A, et al.: Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology 2002, 36:729-736.
32.Marbet UA, et al.: Long-term histological evaluation of the natural history and prognostic factors of alcoholic liver disease. J Hepatol 1987, 4:364-372.
33.Cromie SL, et al.: Chronic hepatitis C: effect of alcohol on hepatitic activity and viral titre. J Hepatol 1996, 25:821-826.
34.Loguercio C, et al.: Drinking habits of subjects with hepatitis C virus-related chronic liver disease: prevalence and effect on clinical, virological, and pathological aspects. Alcohol Alcohol 2000, 35:296-301.
35.Sherman KE, et al.: Hepatitis cRNA quasispecies complexity in patients with alcoholic liver disease. Hepatology 1999, 30:265-270.
36.Monto A: Hepatitis C and steatosis. Semin Gastrointest Dis 2002, 13:40-46.
37.Walsh MJ, et al.: Steatosis and liver cell apoptosis in chronic hepatitis C: a mechanism for increased liver injury. Hepatology 2004, 39:1230-1238.
38.Rigamonti C, et al.: Moderate alcohol consumption increases oxidative stress in patients with chronic hepatitis C. Hepatology 2003, 38:42-49.
39.Szabo G, et al.: Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFN gamma levels. Alcohol Clin Exp Res 2001, 25:1766-1772.
40.Chiappelli F, et al.: Alcohol modulation of human normal T-cell activation, maturation, and migration. Alcohol Clin Exp Res 1995, 19:539-544.
41.* Encke J: Ethanol inhibition: the humoral and cellular immune response to hepatitis C virus NS5 protein after genetic immunization. Alcohol Clin Exp Res 2000, 24:1063-1069.
An interesting demonstration, in a mouse model, of how alcohol intake may play a role in HCV-specific immune function.
42.Geissler M: Inhibitory effects of chronic ethanol consumption on cellular immune response to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmid. J Immunol 1997, 159:5107-5113.
43.National Institutes of Health Consensus Development Conference Panel statement: management of hepatitis C [no authors listed]. Hepatology 1997, 26:2S-10S.
44.** National Institutes of Health Consensus Development Conference statement: management of hepatitis C: 2002. Hepatology 2002, 36:S3-S20.
A fine summary of the current understanding of the pathogenesis of hepatitis C infection and current guidelines for the management of hepatitis C.
45.Marco A, et al.: Predictors of adherence to tuberculosis treatment in a supervised therapy programme for prisoners before and after release. Study Group of Adherence to Tuberculosis Treatment of Prisoners. Eur Respir J 1998, 12:967-971.
46.Dusheiko G: Side effects of alpha interferon in chronic hepatitis C. Hepatology 1997, 26:112S-121S.
47.McHutchison JG, et al.: Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998, 339:1485-1492.
48.Manns MP, et al.: Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C. Lancet 2001, 358:958-965.
49.Fried MW, et al.: Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002, 347:975-982.
50.Muir AJ, et al.: Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med 2004, 350:2265-2271.
51.Oshita M, et al.: Increased serum hepatitis C virus RNA levels among alcoholic patients with chronic hepatitis C. Hepatology 1994, 20:1115-1120.
52.Okazaki T, et al.: Efficacy of interferon therapy in patients with chronic hepatitis C. Comparison between nondrinkers and drinkers. Scand J Gastroenterol 1994, 29:1039-1043.
53.Ohnishi K, et al.: Interferon therapy for chronic hepatitis C in habitual drinkers: comparison with chronic hepatitis C in infrequent drinkers. Am J Gastroenterol 1996, 91:1374-1379.
54.Tabone M, et al.: Alcohol abstinence does not offset the strong negative effect of lifetime alcohol consumption on the outcome of interferon therapy. J Viral Hepat 2002, 9:288-294.
55.Schaefer M, et al.: Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology 2003, 37:443-451.
|
|
| |
| |
|
|
|