| |
Co-occurring Hepatitis C, Substance Use, and Psychiatric Illness: Treatment Issues and Developing Integrated Models of Care
|
| |
| |
Journal of Urban Health: Bulletin of the New York Academy of Medicine, Dec 2004 Vol. 81, No. 4,
Diana L. Sylvestre, Jennifer M. Loftis, Peter Hauser, Sander Genser, Helen Cesari, Nicolette Borek, Thomas F. Kresina, Leonard Seeff, and Henry Francis
Dr. Sylvestre is with the Department of Medicine, University of California, San Francisco; Drs. Loftis and Hauser are with the School of Medicine, Oregon Health and Sciences University, and the Northwest Hepatitis C Resource Center, Portland VA Medical Center; Drs. Genser, Borek, Kresina, and Francis and Ms. Cesari are with the Center for AIDS and Other Medical Consequences of Drug Abuse, National Institute on Drug Abuse, National Institutes of Health; Dr. Seeff is with the National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health.
Correspondence: Thomas F. Kresina, PhD, Center for AIDS and Other Medical Consequences of Drug Abuse, National Institute on Drug Abuse, National Institutes of Health, 6001 Executive Boulevard, MSC 9593, Bethesda, MD 2089209593. (E-mail: Tk13v@nih.gov)
ABSTRACT
Hepatitis C virus (HCV) infection is transmitted by injection drug use and associated with psychiatric conditions. Patients with drug use or significant psychiatric illness have typically been excluded from HCV treatment trials noting the 1997 National Institutes of Health Consensus Statement on HCV that indicated active drug use and major depressive illness were contraindications to treatment of HCV infection. However, the 2002 NIH Consensus Statement recognized that these patients could be effectively treated for HCV infection and recommended that treatment be considered on a case-by-case basis. Treating HCV infection in these patients is challenging, with drug use relapse possibly leading to psychosocial instability, poor adherence, and HCV reinfection. Interferon therapy may exacerbate preexisting psychiatric symptoms. Co-occurring human immunodeficiency virus or hepatitis B virus provide additional challenges, and access to ancillary medical and psychiatric services may be limited. Patients with co-occurring HCV infection, substance use, and psychiatric illness can complete interferon treatment with careful monitoring and aggressive intervention. Clinicians must integrate early interventions for psychiatric conditions and drug use into their treatment algorithm. Few programs or treatment models are designed to manage co-occurring substance use, psychiatric illness, and HCV infection and therapy. The National Institute on Drug Abuse convened a panel of experts to address the current status and the long-range needs through a 2-day workshop, Co-occurring Hepatitis C, Substance Abuse, and Psychiatric Illness: Addressing the Issues and Developing Integrated Models of Care. This conference report summarizes current data, medical management issues, and strategies discussed.
ARTICLE TEXT
Current surveillance data indicate that acute hepatitis C virus (HCV) infection has diminished in the United States partly because of enhanced prevention and testing efforts among injection drug users (IDUs) and blood donors.2 HCV transmission continues primarily through injection drug use (approximately 65%--70% of cases), sex with infected or multiple partners (approximately 15%--20% of cases), or occupational exposure (approximately 5% of cases). The prevalence of HCV infections in the United States is high, with approximately 3 to 4 million Americans estimated to be infected.
Chronic HCV infection is the leading cause of cirrhosis and hepatocellular carcinoma in the United States and the dominant reason for liver transplantation.2 Studies demonstrated that up to 20% of chronically infected patients will develop cirrhosis over a 20-year period, and these patients are at risk for hepatocellular carcinoma. Persons at special risk for progression include those older at the time of infection, alcoholic, or co-infected with human immunodeficiency virus (HIV) or hepatitis B virus. Conversely, individuals infected at a younger age have a far lower rate of disease progression.
Clinical trials showed that the combination of interferon (IFN) and ribavirin was more effective than IFN alone for any genotype, but that the greatest therapeutic yield came with the use of pegylated IFN plus ribavirin.2 Genotype 1, the most common HCV variant in the United States, is less responsive to treatment than genotypes 2 and 3. A sustained virologic response (SVR) is also less common in patients with higher HCV ribonucleic acid (RNA) levels or more advanced stages of fibrosis, especially cirrhosis, and in individuals with HIV/HCV co-infection.3 Persons with genotype 1 who fail to achieve an early virologic response (<2 log decrease in HCV RNA at week 12 of treatment) rarely develop an SVR.4 Initial studies of combination therapy in IDUs, patients with comorbid psychiatric illness, and children indicated that an SVR can be achieved in these populations.
Although the incident data for acute HCV infection in drug users has declined, drug use incidence has not. The prevalence of heroin use has grown over the past decade, with increasing numbers of users presenting for substance use treatment. The National Institute on Drug Abuse and the Community Epidemiology Work Group,5 using ethnographic research, focus groups, and community-based sources, reported that heroin abuse indicators increased nationally in 2002, particularly among young Caucasian and suburban populations. Primary heroin treatment admissions increased in Chicago, Illinois; San Diego, California; Boston, Massachusetts; and Newark, New Jersey, and continued to account for a large proportion of admissions in Baltimore, Maryland; Los Angeles, California; and New York City. In Denver, Colorado, and Dallas, Texas, heroin-related calls to the regional poison control center increased, heroin-related deaths were reported in Detroit, Michigan; Honolulu, Hawaii; Newark; Minneapolis/St. Paul, Minnesota; San Diego, California; and Seattle, Washington. Opiate-related deaths surpassed cocaine deaths in Washington, DC, and Minneapolis/St. Paul.
National seroprevalence studies have shown that exposure to HCV occurs in up to 90% of IDUs.6 For IDUs in methadone maintenance treatment, as many as 96% of tested individuals were seropositive for HCV antibody, and 62% were positive for HCV RNA.7 This compares to prevalence data from cohort studies of drug users who do not inject drugs, with the highest prevalence data reported at approximately 40% for individuals with more than 21 years of drug use.8 Studies have shown that HCV seroconversion in IDUs can occur rapidly in the initial 1--3 years of drug use.9 Thus, the prevalence of HCV infection in IDUs remains high.
Psychiatric disorders are also associated with HCV infection and injection drug use. Recent studies have shown that many patients with HCV infection also have diagnoses of depression, posttraumatic stress disorder (PTSD), psychosis, or anxiety.10--13 Compared to the overall population, individuals with chronic HCV infection have a higher prevalence of psychiatric disorders. For example, in a sample of 931 persons receiving treatment at inpatient and outpatient public health settings in four Eastern states, the prevalence of HCV infection was 19.6%, or 11 times greater than the rates observed in the general population. Injection drug use and use of crack cocaine were risk factors for HCV infection, and a lifetime history of injection drug use was associated with a 31-fold increased risk of HCV infection. Similarly, there is a 6% to 44% prevalence of preexisting depression among HCV-infected patients.14,15
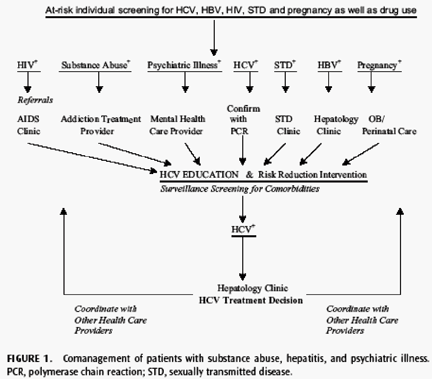
Taken together, these reports indicated that substance use and psychiatric disorders in patients with HCV infection are common and could be significant barriers to treatment of HCV infection. In the following sections, strategies for addressing the issues of co-occurring HCV, substance abuse, and psychiatric illness are presented. Although IFN can lead to severe neuropsychiatric side effects, the published evidence suggested that many patients with psychiatric or substance use diagnoses can be treated safely and effectively. Figure 1 provides a flow diagram that illustrates the comanagement and integration of care for patients with HCV, substance use disorders, and psychiatric illnesses.
|
|
| |
| |
 |
|
| |
| |
SCREENING AND ASSESSMENT OF PSYCHIATRIC, SUBSTANCE USE, AND NEUROCOGNITIVE DISORDERS
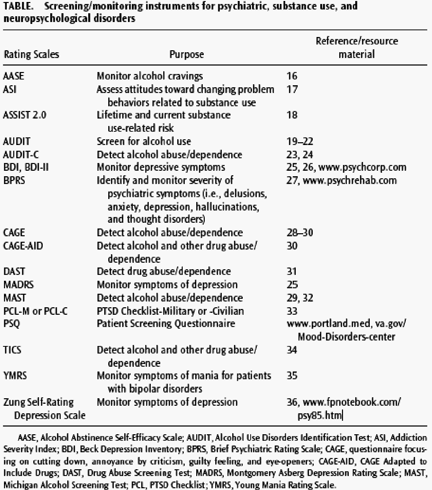
Substance users have a high frequency of co-occurring psychiatric illness. Psychiatric screening and consultation should be considered prior to initiating IFN therapy. For the purposes of screening and monitoring patients for depression before and during IFN therapy, a number of self-rated or clinician-rated scales are available and are listed in the Table. Commonly used self-rated instruments are the Zung Self-Rating Depression Scale,36 the Beck Depression Inventory (BDI),37 and the self-rated version of the Montgomery Asberg Depression Rating Scale.25 A recent report38 examined the sensitivity and specificity of the BDI and other instruments for predicting eventual psychiatric and antidepressant treatment during IFN therapy. The BDI performed best in this analysis and is now the preferred screening tool at Veterans Affairs Medical Centers. These cost-effective tools can provide increased accuracy in assessing
|
|
| |
| |
 |
|
| |
| |
patient depressive symptomatology as well as improved objective measurement of changes in mood states during IFN therapy.39
Gender-specific definitions of levels of hazardous alcohol consumption have been established,28 but are not specific to HCV infection or treatment with IFN. However, heavy alcohol is a key factor in disease progression for both HCV infection and HIV/HCV co-infection. Thus, screening for alcohol consumption in multimorbid patients is imperative. The CAGE questionnaire, focusing on cutting down, annoyance by criticism, guilty feeling, and eye-openers, or the Michigan Alcoholism Screening Test (MAST) can be utilized. However, the Alcohol Use Disorders Screening Test (AUDIT) and the abbreviated version, the AUDIT-C, are best for identifying hazardous use or alcohol use disorders and can be used for stratification of risk to guide prevention and treatment strategies.19,20,23,29 Because of minimization and denial, the AUDIT-C does not measure actual alcohol exposure. Biomarkers of alcohol use have been used, such as carbohydrate-deficient transferrin, hemoglobinassociated aldehyde, and fatty acid ethylesters (in hair),40,41 but there are no sensitivity, specificity, or risk-level standards for HCV-infected populations.
Brief screens for other drug use problems, including the Drug Abuse Screening Test (DAST), CAGE Adapted to Include Drugs (CAGE-AID), and the Two-Item Conjoint Screen (TICS) and tests for drugs in urine, blood, or saliva focus on endstage problems (consequences rather than use) and have no specific focus on tobacco or injection drug use. The ASSIST 2.1 (Alcohol, Smoking, and Substance Involvement Screening Test)18, developed by World Health Organization for primary care screening, assesses (1) lifetime use of 10 drug "classes," (2) substancespecific recent use, (3) substance-specific severity, (4) current global risk, and (5) injection drug risk and shows reliability in international trials. However, its limitations include self-report, length, frequency assessment of alcohol use, and the need for further validation with biomarkers. The Patient Screening Questionnaire (PSQ) has been developed to identify psychiatric and substance use disorders prior to the start of antiviral therapy. This 10-item instrument screens for psychotropic medication use, past psychiatric in- and outpatient care, as well as past substance use.42
Analog scales have been used to assess substance use, psychiatric disease, and cognitive performance. In an ongoing study to assess the combined effects of methamphetamine,
HIV infection, and neurocognitive functioning, the individuals who were co-infected with HCV showed higher levels of cognitive impairment and markers of immune activation than those who were HCV seronegative. In this study, individuals with impaired memory also had higher HCV plasma RNA levels. Although no HCV RNA was found in cerebrospinal fluid (CSF), higher plasma HCV RNA correlated with a higher CSF HIV viral load. Currently, one individual with HIV/HCV co-infection treated with IFN alone showed a decline in the CSF HIV viral load. This is consistent with possible additive or interactive HCV and HIV contributions to neurocognitive impairment in these co-infected substance-using patients. Additional screening tools may be required to assess the individual and combined neurocognitive comorbidities caused by (1) HIV infection, (2) progression of liver disease, and (3) chronic alcohol consumption.
For patients with co-occurring illnesses, there is also the need for assessing function in areas such as driving, employment, self-care, risky behaviors, and adherence. Lessons learned from HIV-infected patients in these areas suggested that neuropsychological (NP) impairment is associated with lower employment percentage and decreased probability of receiving antiretroviral treatment. NP test results can be used to monitor response to treatment.43 For HCV infection, autopsy and imaging studies are providing evidence of HCV viral replication in the brain and abnormal metabolites consistent with central nervous system inflammation in the striatum and white matter,13 suggesting an extrahepatic manifestation of HCV infection that may contribute to neurocognitive impairment. On standard NP tests, HCV-infected patients showed defects of mental and motor speed, "complex" attention (sustained or divided), and short-term working memory, but not IQ or episodic memory.44 This is similar to results found in other patients with cirrhotic or noncirrhotic liver disease, but there are indications that problems emerge earlier among patients with HCV infection.45
The cognitive functioning of HCV-infected drug users has not been characterized. Studies with this population of patients need to consider drug use patterns, level of sobriety or withdrawal when tested, and the lack of test norms that reflect race, ethnicity, socioeconomic status, and frequent comorbidities of drug use (head trauma, learning disorders, malnutrition, HIV, other central nervous system infections, depression, antisocial personality disorder, PTSD). Moreover, classical NP tests such as the Trail-Making Test may provide evidence of impairment without localization or etiology. Computerized tests based on developments in cognitive neuroscience and mapping neuropathology have been useful with HIV-positive patients and may be applicable to those with HCV infection.46 Such tests address (1) verbal working memory (with circuitry including the dorsolateral prefrontal cortex and striatum); (2) decision-making parameters such as risk, impulsivity, and delayed versus immediate reward; (3) mental speed; and (4) divided attention. These tests showed significant correlations with HIV infection and, in the case of verbal working memory, cytokine activity in polydrug users. Data from drug users grouped by HCV infection and HIV serostatus using a reaction time version of the Stroop task to evaluate the integrity of the prefrontal cortical systems mediating response inhibition showed greater Stroop interference in the co-infected group compared with those without infection.47 In addition, the HCV monoinfected patients responded more slowly than did those who were HCV negative. However, studies are needed in HCV-infected drug users whose drug/use history and many comorbid conditions are well characterized to develop practice, brief, and informative assessment of neurocognitive function in patients with HCV infection and co-occurring illnesses.
HEPATITIS C VIRUS TREATMENT AND SUBSTANCE USERS: MANAGEMENT CHALLENGES
Most care providers withhold IFN-based treatment for active drug users who are chronically HCV infected.48 However, calls for an individualized approach to treating HCV-infected patients2,49,50 are based on a limited number of studies that revealed HCV treatment efficacy in drug-using cohorts. A study of 50 heroin addicts enrolled in an inpatient methadone detoxification unit showed an overall SVR of 36%, even though half of the patients relapsed to heroin injection.51 Although the SVR was lower (24%) in the drug users, outcomes were not statistically different from those who maintained sobriety. Only 2 of the 50 patients developed IFN-induced severe depression. The impact of a history of psychiatric and substance use disorders on HCV treatment was studied in 33 veterans; 19 had a history of psychiatric disease, 5 of the 14 nonpsychiatric patients had a remote history of injection drug use, and 13 of the 19 psychiatric patients reported a history of drug or alcohol dependence.52 Psychiatric diagnoses included PTSD, depression, schizoaffective disorder, obsessive-- compulsive disorder, anxiety, and mixed personality disorder. Although patients with psychiatric disease were more likely to develop neuropsychiatric adverse events caused by IFN-based treatment, their dropout rate and SVR were equivalent to those without psychiatric disease. However, virological response rates were low in both groups, and only 30% completed a year of IFN therapy. Overall, 5 patients (2 in the nonpsychiatric cohort and 3 in the psychiatric cohort) developed neuropsychiatric side effects requiring treatment discontinuation.
In a more recent study53 of HCV treatment in 50 methadone patients, 62% (46% depression, 12% anxiety, 2% obsessive compulsive disorder, and 2% schizophrenia) reported a preexisting psychiatric diagnosis, and the end-of-treatment response (ETR) rate was 64%. Of the subjects, 30% were sober for less than 6 months, and 36% relapsed to hard drug use during the study. Pretreated with antidepressants was given to 47% of patients, and 88% were taking antidepressants by the end of IFN therapy. There was no significant difference in ETR between subjects with short sobriety compared to those whose sobriety was more lengthy, and relapse to drug use overall did not lead to a significant reduction in treatment outcomes. Although the dropouts in the study had a modestly higher prevalence of preexisting psychiatric disease than the entire study population (73% vs. 62%, respectively), there was no statistically significant correlation of psychiatric illness with treatment discontinuation.
Furthermore, a long-term follow-up of 27 former IDUs54 treated for HCV infection indicated a limited likelihood of HCV reinfection because of recidivism. Although 33% of the patients returned to injection drug use, only 1 became reinfected, with a defined risk factor of sharing equipment. Taken together, these data warrant the use of the current standard of care, pegylated IFN, for drug users and methadone patients in clinical trials to expand the outcome data of these preliminary studies.
HEPATITIS C VIRUS TREATMENT, EVALUATION, AND MANAGEMENT OF PSYCHIATRIC AND SUBSTANCE USE COMORBIDITY
A growing number of studies provided preliminary support for the use of IFN-based therapy for patients with HCV infection who have active psychiatric illnesses and substance use disorders.38,49,51,53--55 These studies showed that patients with current and past histories of significant psychiatric illness, including substance use disorder, can successfully complete a course of IFN therapy, and that SVR rates were similar to those without such difficulties. Furthermore, patients with a past history of major depressive disorder or substance use may not be at increased risk for the development of IFN-induced depression.56 Further studies are needed to detail definitively the treatment efficacy of IFN-based treatments for patients with HCV, substance use disorders, and psychiatric illnesses.
Given the very high frequency of co-occurring psychiatric and substance use disorders among patients with HCV infection and that antiviral therapy may exacerbate symptoms of psychiatric illness, psychiatric screening strategies for all patients represent optimal care (see Screening and Assessment of Psychiatric, Substance Use, and Neurocognitive Disorders; Table). Regular monitoring of psychiatric symptoms during IFN therapy is also important. The Department of Veterans Affairs Medical Center treatment recommendations for individuals with HCV infection state that patients not exhibiting depression before IFN therapy should be evaluated for depression at least monthly. Patients with depression scores on the BDI, for example, indicating moderate-to-severe depression should be considered for antidepressant treatment and preferably followed by a mental health professional.57
For patients with a history of substance use disorders, regular monitoring using rating scales such as the Addiction Severity Index (ASI) or Alcohol Abstinence Self- Efficacy Scale (AASE) and coordination of care with addiction specialists are also recommended. In patients with co-occurring HCV infection and substance use disorders, possible IFN-induced drug cravings and the risk for drug relapse could seriously compromise their treatment and recovery (see Neurochemistry of the Addicted, Psychiatrically Ill, and Hepatitis C Virus--Infected Patient). A comanagement model of care that uses frequent psychiatric and substance use symptom monitoring and early intervention for reemergence or development of neuropsychiatric side effects and substance use is advocated (Fig. 1).
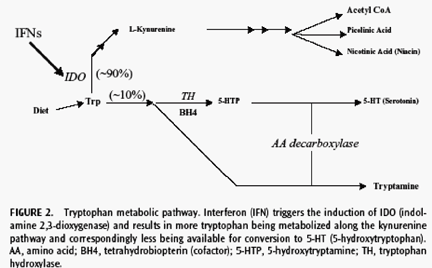
Several reports suggest that antidepressants, primarily selective serotonin reuptake inhibitors (SSRIs), are efficacious in ameliorating IFN-induced major depression in patients with HCV infection.56,58--60 SSRIs can alleviate IFN-induced depression, fatigue, and anxiety as well as cognitive and behavioral slowing.61,62. Importantly, increased depression following IFN therapy is related to the depletion of serum serotonin (Fig. 2) thus, SSRIs may act on the specific neurochemical targets (particularly serotonin), mediating these depressive side effects (see Neurochemistry of the Addicted, Psychiatrically Ill, and Hepatitis C Virus--Infected Patient). However, there are currently no large, placebo-controlled studies that have investigated the use of antidepressant treatment for IFN-induced depression in patients with HCV infection. In addition to using antidepressant treatment to manage preexisting and IFN-induced depression, the use of antidepressants in conjunction with IFN therapy may also serve to decrease relapse potential in patients with substance use disorders. Although the literature is not in complete agreement, treatment with antidepressant medications has been shown to reduce the risk of drug relapse in patients with a history of substance use disorders.63--65 Additional studies are needed to understand better the effects of substance use and psychiatric illness on IFN treatment outcomes in patients with HCV infection.
|
|
| |
| |
 |
|
| |
| |
To improve access to antiviral therapy for patients with co-occurring HCV infection and psychiatric and substance use disorders, future research should identify behavioral and pharmacological interventions that will enhance adherence to antiviral therapy and reduce the reemergence of psychiatric symptoms and substance use.
NEUROCHEMISTRY OF THE ADDICTED, PSYCHIATRICALLY ILL, AND HEPATITIS C VIRUS--INFECTED PATIENT
The majority of cases of HCV infection occur in IDUs, a substantial fraction of whom have current neurocognitive dysfunction or a history of psychiatric illness. The development of addiction, which has a neurochemical basis and is characterized by states of drug craving and extended abstinence, has an impact on several separate, yet interrelated neurobiological processes.66 In animal models of addiction, changes in dopaminergic neurotransmission within a highly limited band of structures, including specific parts of the nucleus accumbens, amygdala, and prefrontal cortex, typically underlie the motivational effects associated with dependence. 67--70 Changes in the signals mediated by several neurotransmitters, including dopamine, opioid peptides, and corticotropin-releasing factor, and in the regulation of transcription factors within the neurons of this reward circuit may underlie the vulnerability to relapse that characterizes addiction in humans.68,71,72 In addition, alterations in gene expression appear to be involved in the long-term neuroadaptive changes associated with the motivational aspects of drug dependence.73
Overlapping and additive neurochemical mechanisms validate the concern that IFN-based treatment of HCV infection may exacerbate preexisting depression or drug use behaviors. In animal models and human studies, there is a direct role for proinflammatory cytokines in the development of depression. Specifically, current literature favors the hypothesis that a relative serotonin depletion plays a central role in IFN-induced depression.74,75 As shown in Fig. 2, the neurochemical events that mediate IFN-related depression are a metabolic increase in the catabolism of tryptophan, specifically through IFN stimulation of the enzyme indoleamine 2,3-dioxygenase, leading to the peripheral depletion of tryptophan, a serotonin precursor.76,77 Thus, the etiology of IFN-induced depression may derive partly from the antiserotonergic effects of IFN, which is consistent with the large body of evidence pointing to a general link between serotonin and depressive disorders, as well as the efficacy of SSRI antidepressants in treating IFN-induced depression (see HCV Treatment, Evaluation, and Management of Psychiatric and Substance Use Comorbidity). However, the mechanism by which IFN produces depression remains unclear and is most probably multifactorial. IFN also modulates neurochemical pathways of other cytokines, the hypothalamicpituitary-adrenal (HPA) axis, and hypothalamic-pituitary-thyroid axis as well as dopaminergic, nonadrenergic, and cholinergic neurotransmission.78--81 IFN increases interleukin 6 (IL-6) production, which correlates with symptoms of depression and anxiety.82 IFN enhances levels of IL-1 and tumor necrosis factor-α (TNF-α), which influences noradrenergic activity. IL-1, IL-6, and TNF-α are also potent stimulators of the HPA axis.84,85 These neurochemical changes may occur in the presence of elevated glucocorticoid concentrations, and immune activation is associated with a decrease in the sensitivity of the glucocorticoid receptors in patients with major depression, thus contributing to altered regulation of the HPA axis.85
Taken together, these observations suggested that IFN-mediated alterations in the HPA axis and perturbations in neurotransmission, especially those related to dopamine or serotonin, may have an additive impact on drug users with depression who are treated for HCV infection. However, whether these neurological events will lead to disproportionately destabilizing neurochemical changes or increase the vulnerability to reinstitution of drug taking remains unclear.
INTEGRATING HEPATITIS C VIRUS TREATMENT INTO HEALTH CARE VENUES
The medical management of HCV infection in multimorbid patients necessitates the integration of IFN-based treatment into health care settings that care for substanceusing and psychiatrically ill patients (Fig. 1). The panel identified the following as venues to address IFN-based treatment of HCV through clinical trials and health services research.
Methadone Clinics
Former IDUs comprise a population that has a high prevalence of HCV infection. Seroprevalence rates range from 67% to 96%, with noninjection (opium smokers) populations exhibiting significantly lower seropositive prevalence rates. Although studies have begun to test the safety, tolerability, and efficacy of antiviral treatment for HCV infection in patients on methadone maintenance, they have excluded active drug users and individuals with severe psychiatric illness or decompensated liver disease. Methadone clinics represent a unique resource to provide screening for HCV infection and psychiatric illness, thereby identifying potential candidates for treatment of substance use and HCV comorbidities.
Prisons
No national surveillance and no systematically collected national data are available regarding HCV among inmates. Based on Centers for Diseases Control and Prevention estimates, assuming needle sharing as the primary risk factor, between 17% and 21% of current inmates are infected with HCV.86 Medical care for inmates is episodic and not given in the context of public health. Therefore, there is no consistent consensus on the treatment of HCV in correctional settings. Some prison systems have developed guidelines for the management of HCV that are not necessarily implemented. Incarcerated individuals, who exhibit drug addiction and psychiatric illness, can be effectively treated for HCV infection with IFN-based therapies. On release, the HCV-infected individual has an impact on the community in terms of disease transmission, costs for medical care, and recidivism. Prevention and treatment interventions would benefit inmates, their families, their partners, and the public health of communities to which inmates return.
Walk-In Clinics
A unique group model that operates as a walk-in clinic for addiction-related HCV has been operating in Oakland, California, since 1998. It was specifically structured to reduce barriers to HCV diagnosis and treatment in less-stable drug users. The clinic is held three times weekly, and its focus is on peer support and interactive education about HCV diagnosis and the treatment process. Attendees are offered on-site blood testing, referrals for biopsy, and comprehensive HCV treatment for interested participants as warranted. To date, over 1,500 low-income addicts have been screened in this cost-effective program, and over 200 have been treated; treatment outcomes appear to be only modestly lower than for nonaddicted patients despite the complexities of the patients served.
Community Mental Health Clinics and Psychiatric Hospitals-
The HCV treatment for persons with severe mental illness (SMI) in public mental health settings presents unique challenges and opportunities, including medical, psychosocial, housing, daily living, legal, and vocational services. Models of care that integrate mental health and substance use treatment can be effective,87,88 but are not routinely implemented because of limited financial and human resources.24 Guidelines to treat HCV in persons with SMI are not available, and there are no national HCV guidelines for persons receiving treatment at community mental health centers or state psychiatric hospitals. Care practices vary greatly by state and community, and HCV-related service needs are not uniformly addressed. Models of care developed for community-based psychiatric care of persons with SMI, including assertive community treatment (ACT) and intensive case management, can provide a valuable framework for treating HCV in public mental health settings. ACT teams provide a range of services, such as sexual education, reproductive counseling, and medication support services.89 These programs can be expanded to include services related to HCV. Intensive case management can also be utilized to monitor symptoms and provide integration of psychiatric and medical teams.
One community model in a mental health clinic setting utilizes a service team, comprised of a nurse, an infectious disease specialist, a psychiatric specialist, and an administrator, who provide education and assist in the development of services, including best practices outlined by the Centers for Disease Control and Prevention for HCV care: screening, testing, immunization, risk reduction counseling and referral, and support for medical care.
SUMMARY AND DIRECTIONS FOR FUTURE RESEARCH
There are vast gaps in the understanding of the optimal management of HCV treatment in substance users and patients with comorbid psychiatric illness. Clinical research and trials are needed to understand further how to select patients who can successfully undergo therapy and to define better the impact of intervening drug use, the minimally appropriate length of sobriety, and the impact and management of the spectrum of preexisting psychiatric conditions. Information is needed on interventions when relapse to substance use occurs and whether different drugs of abuse present unique outcomes. Clinical research is needed for a better understanding of the individual and cumulative impact of the potential barriers to HCV treatment in patients with co-occurring HCV, substance abuse, and psychiatric illness, as well as for new approaches to the medical management of these patients and novel interventions that promote a SVR to HCV treatment.
Existing data suggest that selected substance users can be candidates for HCV treatment, even in the setting of psychiatric disease and relapse to drug use. In light of the vast impact of HCV in this population and their limited access to HCV treatment and life-saving liver transplantation, the urgent need for further clinical study is clear.
ACKNOWLEDGEMENT
Co-occurring Hepatitis C, Substance Use and Psychiatric Illness: Addressing the Issues and Developing Integrated Models of Care took place December 5--6, 2003, in Bethesda, Maryland. The conference was sponsored by the Center for AIDS and Other Medical Consequences of Drug Abuse (CAMCODA); National Institute of Drug Abuse and Division of Digestive Diseases and Nutrition; National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK); National Institutes of Health; Department of Health and Human Services; and Veterans Administration.
REFERENCES
1. Hoofnagle JH, Tralka TS. Introduction: The National Institutes of Health Consensus Development conference: management of hepatitis C. Hepatology. 1997;26(suppl 1):1S.
2. Seeff LB, Hoofnagle JH. Appendix: the National Institutes of Health Consensus Development Conference Management of Hepatitis C 2002. Clin Liver Dis. 2003;7:261--287.
3. Sherman KE. Implications of peginterferon use in special populations infected with HCV. Semin Liver Dis. 2003;23(suppl 1):47--52.
4. Zeuzem S, Heathcote EJ, Shiffman ML, et al. Twelve weeks of follow-up is sufficient for the determination of sustained virologic response in patients treated with interferon alpha for chronic hepatitis C. J Hepatol. 2003;39:106--111.
5. Community Epidemiology Work Group. Epidemiologic trends in drug abuse. In: Proceedings of the Community Epidemiology Work Group December 2001. Ed. Moira O'Brian. Vol. 1. Bethesda, MD: National Institute on Drug Abuse, NIH, DHHS; 2002:1--68. NIH Publication 02--5109.
6. Patrick DM, Buxton JA, Bigham M, Mathias RG. Public health and hepatitis C. Can J Public Health. 2000;91(suppl 1):S18--S23.
7. McCarthy JJ, Flynn N. Hepatitis C in methadone maintenance patients: prevalence an public policy implications. J Addict Dis. 2001;20:19--31.
8. Quaglio G, Lugoboni F, Pajusco B, et al. Facors associated with hepatitis C virus infection in injection and noninjection drug users in Italy. Clin Infect Dis. 2003;37:33--40.
9. Hagan H, Des Jarlais DC. HIV and HCV infection among injection drug users. Mount Sinai J Med. 2000;67:423--428.
10. Osher FC, Goldberg RW, McNary SW, Essock SM, Butterfield MI, Rosenberg SD. Substance abuse and the transmission of hepatitis C among persons with severe mental illness. Psychiatr Serv. 2003;54:842--847.
11. Klinkenberg WD, Caslyn RJ, Morse GA, et al. Prevalence of human immunodeficiency virus, hepatitis B, and hepatitis C among homeless persons with co-occurring severe mental illness and substance use disorders. Compr Psychiatry. 2003;44;293--302.
12. Zdilar D, Franco-Bronson K, Buchler N, Locala JA, Younossi ZM. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207--1211.
13. Forton MD, Taylor-Robinson SD, Thomas HC. Cerebral dysfunction in chronic hepatitis C infection. J Viral Hepat. 2003;10:81--86.
14. Miyaoka H, Otsubo T, Kamijima K, Ishii M, Onuki M, Mitamura K. Depression from interferon therapy in patients with hepatitis C. Am J Psychiatry. 1999;156:1120--1129.
15. Pariante CM, Orru MG, Baita A, Farci MG, Carpiniello B. Treatment with interferonalpha in patients with chronic hepatitis and mood or anxiety disorders. Lancet. 1999; 354:131--132.
16. DiClemente CC, Carbonari JP, Montgomery RP, Hughes SO. The Alcohol Abstinence Self-Efficacy scale. J Stud Alcohol. 1994;55:141--148.
17. McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic instrument for substance abuse patients: the Addiction Severity Index. J Nerv Mental Dis. 1980; 168:26--33.
18. WHO ASSIST Working Group. The WHO Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97: 1183--1194.
19. Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro M. AUDIT—the Alcohol Use Disorders Identification Test: guidelines for use in primary health care. World Health Organization, Department of Mental Health and Substance Abuse, 2001. Accessed September 3, 2004. Available at: http://www.who.int/substance_abuse/PDFfiles/auditbro.pdf.
20. Bradley KA, Bush K, Dobie DJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821--829.
21. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423--432.
22. Reinert DF, Allen JP. The alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcohol Clin Exp Res. 2002;26:272--279.
23. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789--1795.
24. Drake RE, Essock SM, Shaner A, et al. Implementing dual diagnosis services for clients with severe mental illness. Psychiatr Serv. 2001;52:469--476.
25. Svanborg PM, Asberg M. A comparison between the Beck Depression Inventory (BDI) and the self-rating version of the Montgomery Asberg Depression Rating Scale (MADRS). J Affect Disord. 2001;64:203--216.
26. Beck AT, Steer RA, Ball R. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588--597.
27. Flemenbaum A, Zimmermann RL. Inter- and intra-rater reliability of the Brief Psychiatric Rating Scale. Psychol Rep. 1973;32:783--792.
28. NIAAA. Helping Patients with alcohol problems: a health practitioners guide. January 2003. NIH Publication 03--3769. Accessed September 3, 2004. Available at: http://www.niaaa.nih.gov/publications/Practitioner/HelpingPatients.htm.
29. Fiellin DA, Reid MC, O'Connor PG: Screening for alcohol problems in primary care. Arch Intern Med. 2000;160:1977--1989.
30. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in primary care practice. Wisc Med J. 1995;94:135--140.
31. Gavin DR, Ross HE, Skinner HA. Diagnostic validity of the drug abuse screening test in the assessment of DSM-III drug disorders. Br J Addict. 1980;84:301--307.
32. Rumpf H-J, Hapke H, Meyer C, John U. Screening for alcohol use disorders and at-risk drinking in the general population: psychometric performance of three questionnaires. Alcohol Alcohol. 2002;37:261--268.
33. Deployment Health Clinical Center. PTSD Checklist—Military Version (PCL-M). Accessed September 3, 2004. Available at: www.pdhealth.mil/guidelines/appendix4.asp.
34. Brown RL, Leonard T, Saunders LA, Papasouliotis O. A two-item conjoint screen for alcohol and other drug problems. J Am Board Fam Pract. 2001;14:95--106
35. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;33:429--435.
36. Zung WW, Wonnacott TH: Treatment prediction in depression using a self-rating scale. Biol Psychiatry. 1970;2:321--329.
37. Beck AT, Ward C, Mendelson M, Mock JE, Erbaugh JK. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561--571.
38. Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104--112.
39. Wright IA. Monitoring depression in patients undergoing alpha-interferon and ribavirin therapy for hepatitis C. Gastroenterol Nurs. 2000;23:275--280.
40. Bean P. Update on new biomarkers for detecting excessive alcohol use. AlcoholMD.com Web site. November 2002. Available at: http://www.alcoholmd.com/pro/courses/biomarkers_of_alcohol_abuse.asp.
41. Wolff K, Farrell M, Marsden J. A review of biological indicators of illicit drug use: practical considerations and clinical usefulness. Addiction. 1999;94:1279--1298.
42. Portland VA Medical Center, Mood Disorders Center. Patient Screening Questions (PSQ). Accessed September 3, 2004. Available at: www.portland.me.va.gov/mood-disorders-center.
43. Martin EM, Pitrak DL, Novak RM, Pursell KJ, Mullane KM. Reaction times are faster in HIV-seropositive patients on antiretroviral therapy: a preliminary report. J Clin Exp Neuropsychol. 1999;21:730--735.
44. Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440--446.
45. Forton DM, Thomas HC, Murphy CA, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35:433--439.
46. Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343--1349.
47. Martin EM, Novak RM, Fendrich M, et al. Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. J Int Neurophysiol Soc. 2004;10:298--300.
48. Stephenson J. Former addicts face barriers to treatment for HCV. JAMA. 2001;285: 1003--1005.
49. Davis GL, Rodrigue JR. Treatment of chronic hepatitis C in active drug users. N Engl J Med. 2001;345:215--217.
50. Edlin BR, Seal KH, Lorvick J, et al. Is it justifiable to withhold treatment for hepatitis C from illicit-drug users. N Engl J Med. 2001;345;211--214.
51. Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001; 34:188--193.
52. Ho SB, Nguyen H, Tetrick LL, Opitz GA, Basara ML, Dieperink E. Influence of psychiatric diagnoses on interferon-alpha treatment for chronic hepatitis C in a veteran population. Am J Gastroenterol. 2001;96:157--164.
53. Sylvestre DL. Treating hepatitis C in methadone maintenance patients: an interim analysis. Drug Alcohol Depend. 2002;67:117--123.
54. Dalgard O, Bjoro K, Hellum K, et al. Treatment of chronic hepatitis C in injecting drug users: 5 years' follow-up. Eur Addict Res. 2002;8:45--49.
55. Van Thiel D, Friedlander L, Molloy P, Fagiuoli S, Kania R, Caraceni P. Interferon-alpha can be used successfully in patients with hepatitis C virus-positive chronic hepatitis who have a psychiatric illness. Eur J Gastroenterol Hepatol. 1995;7:165--168.
56. Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942--947.
57. San Francisco and Miami Centers of Excellence in Hepatitis C Research and Education and the Hepatitis C Technical Advisory Group DoVA. Treatment Recommendations for Patients With Chronic Hepatitis C. Version 1.0. Washington, DC: Veteran's Health Administration, Department of Veterans' Affairs; 2002.
58. Levenson J, Fallon H. Fluoxetine treatment of depression caused by interferon-alpha. Am J Gastroenterol. 1993;88:760--761.
59. Gleason O, Yates W. Five cases of interferon-alpha-induced depression treated with antidepressant therapy. Psychosomatics. 1999;40:510--512.
60. Schramm T, Lawford B, Macdonald G, Cooksley W. Sertraline treatment of interferonalfa- induced depressive disorder. Med J Aust. 2000;173:359--361.
61. Loftis J, Socherman RE, Whitehead AJ, Hauser P. Interferon-alpha-induced depression: time course and antidepressant response of symptom dimensions for patients with hepatitis C. Psychosom Med. In press.
62. Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferonalpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643--652.
63. Pettinati HM. The use of selective serotonin reuptake inhibitors in treating alcoholic subtypes. J Clin Psychiatry. 2001;62:26--31.
64. Naranjo CA, Knoke DM. The role of selective serotonin reuptake inhibitors in reducing alcohol consumption. J Clin Psychiatry. 2001;62:18--25.
65. Srisurapanont M, Kittiratanapaiboon P, Jarusuraisin N. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2001:CD003026.
66. McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689--1695.
67. Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715--723.
68. Wise RA, Bozarth MA. Brain substrates for reinforcement and drug self-administration. Prog Neuropsychopharmacol. 1981;5:467--474.
69. Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223--244.
70. Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247--291.
71. Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23--47.
72. Stimmel B, Kreek MJ. Neurobiology of addictive behaviors and its relationship to methadone maintenance. Mt Sinai J Med. 2000;67:375--380.
73. Nestler EJ, Hope BT, Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995--1006.
74. Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468--473.
75. Bonaccorso S, Marino V, Puzella A, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferonalpha- induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86--90.
76. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3 dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516--2522.
77. Brown RR, Ozaki Y, Datta SP, Borden EC, Sondel PM, Malone DG. Implications of interferon-induced tryptophan catabolism in cancer, auto-immune disease and AIDS. Adv Exp Med Biol. 1991;294:425--435.
78. Boyer P. Do anxiety and depression have a common pathophysiological mechanism. Acta Psychiatr Scand Suppl. 2000;406:24--29.
79. Leonard BE, Song C. Changes in the immune system in rodent models of depression. Int J Neuropsychopharmacol. 2002;5:345--356.
80. Jones TH, Wadler S, Hupart KH. Endocrine-mediated mechanisms of fatigue during treatment with interferon-alpha. Semin Oncol. 1998;25(suppl 1):54--63.
81. Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behavior: implications for "depression due to a general medical condition," immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5:389--399.
82. Bonaccorso S, Puzella A, Marino V, et al. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105:45--55.
83. Foucart S, Abadie C. Interleukin-1 beta and tumor necrosis factor-alpha inhibit the release of 3H-noradrenaline form mice isolated atria. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:1--6.
84. Dunn AJ. Effects of the IL-1 receptor antagonist on the IL-1 and exotoxin-induced activation of the HPA axis and cerebral biogenic amines in mice. Neuroimmunomodulation. 2000;7:36--45.
85. Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60--66.
86. Spaulding A, Lau D, Weinbaum C, et al. Developing a framework for correctional management of hepatitis C. A review of current and future management of the disease in prisons. In press.
87. Drake RE, Mercer-McFadden C, Mueser KT, McHugo GJ, Bond GR. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24:589--608.
88. Druss BG, Rohrbaugh RM, Levinson CM, Rosenheck RA. Integrated medical care for patients with serious psychiatric illness: a randomized trial. Arch Gen Psychiatry. 2001; 58:861--868.
89. Phillips SD, Burns BJ, Edgar ER, et al. Moving assertive community treatment into standard practice. Psychiatr Serv. 2001;52:771--779.
|
|
| |
| |
|
|
|