| |
The management of side-effects during therapy for hepatitis C
|
| |
| |
Alimentary Pharmacology & Therapeutics
Volume 20 Issue 9 Page 917 - November 2004
R. J. Aspinall & P. J. Pockros
Division of Gastroenterology/Hepatology, Scripps Clinic, La Jolla, CA, USA
Summary
The results of interferon and ribavirin combination therapy for chronic hepatitis C infection have substantially improved in recent years, such that the majority of patients in randomized-controlled trials now achieve a sustained virological response. However, adverse effects are commonplace, often disabling and may lead to interruption or cessation of therapy with subsequent loss of efficacy. Constitutional, neuropsychiatric and haematological reactions have proved particularly troublesome. In this review, we discuss these adverse effects in more detail and highlight recent advances in their management.
Introduction
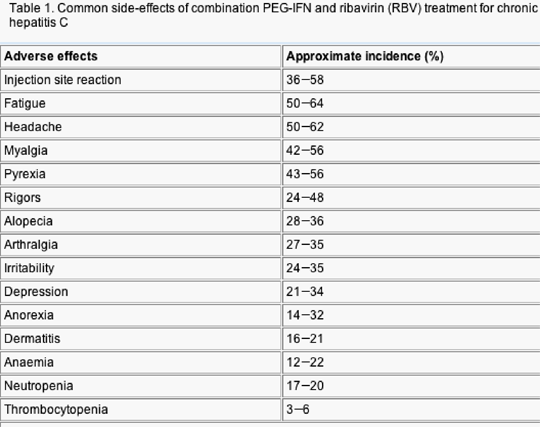
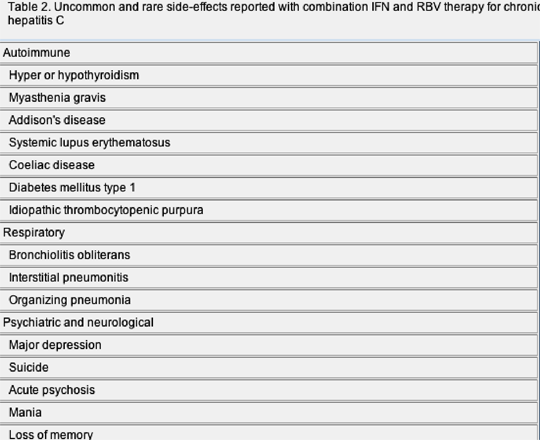
Combination therapy using subcutaneous pegylated interferon (PEG-IFN) alpha -2a or -2b, together with oral ribavirin (RBV) has significantly advanced the treatment of chronic hepatitis C and represents the current standard of care. Several large randomized-clinical trials have now demonstrated that a majority of patients can achieve an sustained virological response (SVR) with these regimens. Maintaining anti-viral pressure, particularly during the initial 12 weeks of therapy, is of paramount importance if the virus is to be cleared. Unfortunately, adverse effects (AEs) related to IFN or RBV are relatively common and may lead to discontinuation of treatment in approximately 10-15% of patients. Most individuals suffer from some side-effects and the most frequent include flu-like symptoms, myalgia, fatigue, gastrointestinal disturbances, psychiatric disorders and haematological abnormalities (Tables 1 and 2). The majority of patients can be managed with supportive measures and/or adjustment of anti-viral dosage, but recent work has highlighted some additional interventions that may help ameliorate adverse reactions and maintain compliance with therapy.
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
General principles
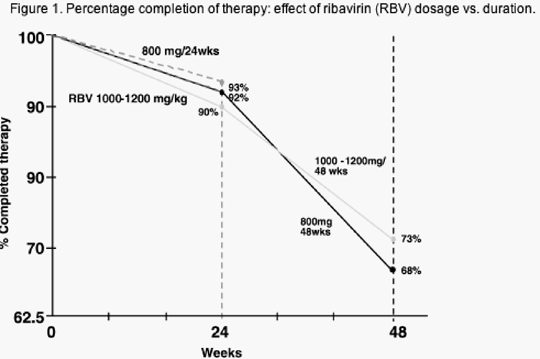
The principles of side-effect management for PEG-IFN and RBV therapy are similar to those we have learned from standard IFN combination (IFN/RBV) therapy. Diminution of AE may occur in some instances. However, unlike standard IFN/RBV therapy we often see a worsening of AEs with time during PEG-IFN/RBV therapy especially after the first 24 weeks of treatment. This fact is clear when one examines the prevalence of dose reduction or discontinuation after 24 weeks compared with after 48 weeks (Figure 1). Therefore, patients must be monitored on a regular basis and dose reductions, drug holidays or discontinuation may be required at any time during treatment.
|
|
| |
| |
 |
|
| |
| |
Patient selection is clearly important. Whereas most clinical trials of anti-viral therapies have carefully excluded individuals with significant psychiatric illness, major medical comorbidity or recidivist substance abuse, the incidence of adverse reactions to PEG-IFN/RBV in these wider patient populations may be significantly higher. The decision to proceed with treatment, as ever, requires a cautious weighing of the risk to benefit ratio for each individual.
As with prior treatment regimens, patients and caregivers should be fully informed about the natural history of hepatitis C, its treatment and the possible side-effects before therapy is commenced. In particular, patients should be aware of the high likelihood of developing symptoms that may, temporarily at least, worsen their quality of life (QoL). Close patient monitoring, prompt access to medical services when necessary and the involvement of a specialist multidisciplinary team are all likely to be of benefit in detecting and managing adverse reactions.
At our treatment centre at Scripps Clinic, we recommend acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs) for flu-like symptoms and fever related to PEG-IFN injections. It is our practice to limit the dosage of acetaminophen to 2 g/24 h, given its altered pharmacokinetics in chronic liver disease. NSAIDs should be avoided in patients with established cirrhosis in view of the recognized risk of precipitating renal impairment.
These constitutional symptoms typically last for 48 h after injection but in many cases they are delayed for 24-48 h before they occur. In addition to the above medication, we also encourage sufficient hydration and adequate sleep. Although there are no specific dietary recommendations for patients undergoing treatment, we generally recommend patients try to remain fit with a body mass index (BMI) <30 and ideally below 25. There is ample evidence that overweight patients have more advanced fibrosis and may have a poorer response to therapy, with some additional evidence of disordered IFN pharmacokinetics in obese individuals.
Management of fatigue has no scientific basis but it seems sensible for patients to plan lighter activities, especially on days after PEG-IFN injections. We typically recommend the injections be given the evening before a weekend, allowing time for a 48-h rest if needed. If fatigue is profound, the patient should be evaluated in the office for other causes such as RBV-induced anaemia (which may occur quite rapidly), electrolyte imbalance and hypothyroidism. At least half of our patients who have profound fatigue are ultimately identified to be suffering from depression, which can be treated with medication and/or psychotherapy (see below). In addition, many of the patients with insomnia and irritability are also found to be depressed and may respond to antidepressant therapy. We find an antidepressant with sedative effects such as trazadone is often useful in such cases, although side-effects of this drug may include priapism and, rarely, hepatotoxicity.
Although there are no published data on the use of stimulant medications for the fatigue related to PEG-IFN/RBV therapy, some practitioners have claimed success with methyephenidate HCL or amphetamine salts for fatigue. Furthermore, although there are no data on the use of appetite stimulants for patients with anorexia related to treatment, we are aware that some patients have benefited from dronabinol. However, caution is advised when using these drugs for such off-label indications.
Interestingly, patients' expectations and their pre-treatment symptomatology appear to have a strong influence on the development of fatigue and other constitutional symptoms during anti-viral therapy. One multivariate analysis of patients given IFN monotherapy reported that the presence of a debilitating side-effect after 6 months of treatment was associated with the presence of the same symptom prior to starting IFN in all cases. Surveyed patients generally judge the magnitude of such symptoms more severely than their doctors and also appear to overestimate the likely chances therapeutic success, suggesting the need for a closer understanding between doctor and patient.
The management of cough associated with therapy is also troublesome. The dry, non-productive cough is usually caused by RBV therapy and typically will not clear until RBV is stopped. Most patients are able to tolerate the cough but in some cases cessation of the RBV is necessary. In cases where the cough becomes productive or is associated with an abnormal auscultatory examination or fever, the patient should undergo chest X-ray to rule-out the possibility of pneumonitis. If bacterial pneumonia is revealed, anti-viral therapy should be withheld until antibiotics have been given and there is clear evidence of clinical improvement.
The management of RBV-induced skin eruptions and pruritis in general is similarly difficult. The rash seen with RBV is usually present on the trunk and back, is macular-papular and pruritic. We have not seen a tremendous response to topical steroid creams or soothing baths. The rash disappears within weeks of stopping RBV. Occasionally, the RBV rash spreads to the face and may be associated with severe periorbital oedema. RBV should be discontinued in such cases.
Injection-site reactions are essentially universal with PEG-IFN therapy. The reaction sites are usually red and slightly raised and may expand to a circumference of 5 cm or more. We recommend rotating injection sites for this reason, as the lesions may take weeks to resolve. If an injection site reaction continues to enlarge or becomes warm and tender, the patient should be examined for the development of an abscess. Where an abscess is promptly diagnosed, we are typically able to drain the site and treat with oral antibiotics without interruption of PEG-IFN therapy. A large abscess should be considered as a potentially severe adverse event and therapy discontinued until it is healed, or even indefinitely.
Because of the potential of ophthalmological adverse events with PEG-IFN therapy, we advocate a formal slit-lamp examination on all patients prior to initiating treatment. This is particularly important in patients at risk for underlying retinal disease such as those with hypertension and/or diabetes mellitus. The ophthalmologist should specifically look for cotton-wool spots and retinal haemorrhage, the two most common effects on the eye seen with PEG-IFN therapy. If the patient experiences any change in vision during treatment they are immediately referred back to the ophthalmologist for a repeat examination in order to determine if there has been a change from the baseline. We always discontinue PEG-IFN therapy until the visual disturbance has resolved or the ophthalmologist has confirmed there is no retinal injury.
Because of the infrequent development of thyroid abnormalities with PEG-IFN therapy it is recommended that a thyroid-stimulating hormone (TSH) level be tested before initiation of treatment, at least once during treatment (we usually do this at week 12) and at any time the patient reports symptoms suggestive of hypo- or hyperthyroidism. Additionally, because of the rare cases of arrhythmia and/or cardiomyopathy that have been associated with PEG-IFN therapy, we typically evaluate a patient's cardiac status prior to therapy with a resting electrocardiogram. If this is abnormal, or if there is a prior history of cardiac disease, we add echocardiography, treadmill examination and cardiology consultation if indicated. Patients experiencing arrhythmias during therapy should be immediately evaluated and treatment held until the verification that no serious event has occurred.
Review of the data from a large multicentre trial evaluating PEGIFN-RBV combination therapy showed equal or worse AEs in almost all areas compared with standard IFN-RBV combination. The most prominent among these AEs were injection site reactions and dose reductions for neutropenia. A similar comparison of adverse events with PEG-IFN alpha -2a/RBV combination therapy shows somewhat less side-effects than standard combination therapy. It is difficult to determine the importance of these trial differences in clinical practice until a significant clinical experience with both compounds has accumulated. It will also not be possible to accurately compare the respective side-effect profiles of these two pegylated compounds with one another until a head-to-head trial is done.
Practical management of neuropsychiatric adverse effects
Depression occurs in up to 37% of patients who receive combination therapy of PEG-IFN and RBV. As such, it is critical to conduct pretherapy assessment of patients for underlying neuropsychiatric conditions including past history of depression, melancholia, hospitalization for suicide attempts and psychoses. In addition, it is critical to inquire about previous alcohol and substance abuse as these are co-morbidities, which often predispose to depression in this patient population. In some cases, a simple family history of depression without past medical history in the cohort is a harbinger of development of depression during therapy. Given that chronic infection with hepatitis C is itself associated with a significant reduction in QoL, 16 it is hardly surprising that low mood is a common finding even before starting anti-viral treatment.
In our centre at Scripps Clinic, we conduct pretherapy Beck Depression Inventory (BDI) testing and routine assessments of the BDI during therapy. We have found this to be an excellent way to alert us of early development of depression, often prior to any history provided by the patient. Many individuals with mild-to-moderate depression are able to manage the side-effects of therapy with the use of antidepressants and dosage adjustment of PEG-IFN during therapy and thus have the opportunity for SVR.
Figure 2 shows the proportion of patients with depression reported in a large multicentre trial, evaluating over 1000 patients with chronic hepatitis C. This data indicates that the cumulative percentage of patients with depression over time treated with PEG-IFN alpha -2a at a weekly dose of 180 mcg plus RBV was significantly less than that of standard IFN plus RBV (21% vs. 30%, P < 0.05). Although this data is somewhat difficult to interpret because of a lack of comparison with other PEGIFN-RBV compounds, it suggests that patients treated with this regimen may be better able to tolerate therapy. In our own clinical experience, we have found that depression may occur in patients receiving PEG-IFN alpha -2a plus RBV later in treatment regimen, often after week 12, whereas symptoms of depression with standard IFN-RBV typically present during the first month or 2 of treatment. This appears to improve adherence to therapy during the critical first 12 weeks of treatment in our patients receiving PEG-IFN alpha -2a combination therapy.
During the patient interview prior to instituting therapy when considering candidacy for anti-viral therapy, one should assess the patient for symptoms or signs of major depression. These would include major mood and hedonic responsiveness, decreased vital sense, decreased self-attitude and in some cases neurovegetative signs such as early morning awakening, appetite change, diminished libido or cognitive impairment. If any or all of these signs or symptoms are present, the patient should be formally evaluated for depression, usually by a psychologist or psychiatrist.
Patients with hepatitis C typically have statistically higher BDI scores when compared with normal population or those with other liver diseases. A similar testing tool, the Hospital Anxiety and Depression Scale (HADS) has shown that 45% of hepatitis C patients screen positive for depression, compared with 4% of healthy controls. The prevalence of depressive symptoms is even higher at around 60% in patients who are HIV-positive active drug users. Therefore, the practitioner must be very aware of the high prevalence of depression in this patient population and actively seek it out prior to the initiation of therapy. If this is not done, inevitably, the patient will manifest features of depression, sometimes in a dramatic way during IFN/RBV or PEG-IFN/RBV treatment. This often results in early suspension or termination of therapy, unnecessary dose reductions and a lost opportunity to attain a SVR.
At our own centre, we have a low threshold for starting patients on selective serotonin reuptake inhibitor (SSRI) antidepressant medications prior to initiation of treatment. We have found this to be an effective strategy that often allows the patient to complete therapy treatment with excellent adherence. We are especially prone to considering this in patients who have previously taken an SSRI but are not currently being treated with the medication during the evaluation period.
The prevalence of depression during treatment ranges from 22 to 44% with an average of approximately a third of patients. In many of the large multicentre registration trials performed for IFN/RBV or PEG-IFN/RBV therapy, irritability and anxiety were reported as separate adverse events from depression. One should be cautioned that these are often features of early depression, which is often masked both to the patient and to the practitioner. BDI testing may be helpful in this setting to clarify the source of the irritability and anxiety.
There are two goals of treatment of IFN-associated depression. First, the alleviation of symptoms and second the completion of IFN therapy, preferably without interruption or dose reduction. Numerous antidepressants have been investigated in successful trials of therapy for IFN-induced depression including Fluoxetine, Sertraline, Citalopram, Paroxetine, Nortriptyline and Imipramine. 26-29 The overall success rate of these agents has been close to 90% in the recorded trials. As such, the main limiting factor of the compounds is their own side-effects. Specifically, tricyclic antidepressants may cause dry mouth, constipation, urinary retention, sedation and weight gain. SSRIs are often associated with sexual dysfunction and some such as Fluoxetine, Sertraline, Citalopram and Paroxetine are associated with anxiety or insomnia. Others such as Paroxetine or Citalopram, are associated with sedation. The drugs may be selected for their side-effect profile in specific individuals where these actions may be desirable. For instance, in patients who are overly fatigued during the day, one may select an SSRI antidepressant, which is stimulating. Similarly, for patients who have difficulty with sleep, one may select an SSRI at bedtime, which has sedative properties.
Other antidepressants have been used for treatment of depression during anti-viral therapy but have not been as well studied. Each of these has their own AE profile. They include Bupropion SR (anxiety, insomnia and nausea), Venlafaxin (activation hypertension), Nefazodone (sedation, anxiety and nausea) and Mirtazepine (sedation and weight gain). In some cases, augmentation of antidepressants may be necessary using lithium salts, tri-iodothyronine or low doses of antipsychotics. These agents should only be deployed by health care professionals with substantial experience in their use.
The efficacy of psychopharmacological management was convincingly demonstrated in one recent prospective trial studying patients with IFN-induced mood disorders. Psychiatric adverse events developed in 30% of patients with psychopharmacological therapy being required in half of those cases. Patients received either antidepressants (for low mood), benzodiazepines (for anxiety) or thioridazine (for irritability) and it was only necessary to discontinue IFN in one of the 60 patients originally enrolled.
It seems reasonable to consider whether IFN-induced depression can be prevented. Prophylaxis has been best studied in patients receiving high doses of IFN for malignant melanoma. In a double-blind placebo-controlled trial, patients given Paroxetine prophylaxis had a significantly lower rate of depression and were less likely to discontinue IFN than a control group. To date, limited data is available regarding prophylaxis in patients being treated for hepatitis C, although large multicentre trials are underway at this time and should be published shortly.
In summary, psychiatric and substance use disorders are extremely common among patients with hepatitis C and in some may be the source of infection to begin with. As such, all patients should be screened for psychiatric morbidity, particularly depression, prior to the initiation of IFN therapy. Furthermore, these patients should be closely monitored, preferably with an objective screening tool such as the BDI or HADS, at regular intervals during therapy. If significant depression is recognized, patients should be appropriately treated with antidepressants and/or formally evaluated by a mental health care worker. Models of care that incorporate the assessment and treatment of psychiatric illness and substance abuse exist.
Management of haematological side-effects
The haematological adverse events of IFN and RBV deserve particular consideration, not least because they were the most common laboratory abnormalities responsible for dose reductions or discontinuations in the major clinical trials. There are three major problems encountered: neutropenia, thrombocytopenia and anaemia.
Neutropenia
Patients receiving PEG-IFN alpha -2a combination or monotherapy experience significant neutropenia in approximately 20% of cases. Dose reductions occurred in monotherapy and combination therapy trials of PEG-IFN alpha -2a and RBV in 17-20% of individuals. Patients receiving PEG-IFN alpha -2b and RBV were noted to have dose modifications for neutropenia in 18% of patients receiving 1.5 mcg/kg of PEG-IFN alpha -2b, the recommended dosage.
The PEG-IFN manufacturers currently recommend dose reduction if the peripheral absolute neutrophic count (ANC) fall below 750 mm3 and blood counts should then be closely monitored until a steady-state is resumed. Currently, dose reduction is the only safe management for neutropenia, although some practitioners have used granulocyte colony-stimulating factor (G-CSF) at a dose of 300 mcg three times a week for correction of neutropenia. There have been no double-blind randomized trials showing this approach to be safe and effective to date. Recent guidelines from the American Association for the Study of Liver Diseases concluded there were currently insufficient data to recommend the routine use of G-CSF as a means of avoiding reductions in drug dosages. However, a number of small reports and anecdotal information from doctors treating co-infected individuals and post-transplant patients have suggested that the use of growth factors for neutropenia can be effective and generally well-tolerated.
Two recent reports indicate that there is no association between neutropenia in this population and the development of bacterial infections. In the largest study report, featuring preliminary data from a still ongoing multicentre trial, there were only 30 of 4243 patients (0.7%) who developed severe infections during anti-viral therapy. When examined closer, the mean ANC nadir in this group was no different than that of the entire treated study population. There was also no difference in the percentage of patients who had ANC < 750/mm3, or was there any association between the ANC nadir and the development of infection. These data suggest that further meticulous studies are required to determine the criteria for dose reduction and to define the use of G-CSF for PEG-IFN-induced neutropenia.
Thrombocytopenia
Interferon therapy frequently results in a 10-50% fall in the platelet count, although this is rarely of clinical significance. Possible mechanisms include a relative thrombopoietin deficiency, impaired thrombopoietin signal transduction in megakaryocytes and in some cases increased immune-mediated sequestration of platelets. During the trial reported by Fried et al., only around 4-6% of patients receiving PEG-IFN alpha -2a and RBV required dose reductions for thrombocytopenia. 3 This was broadly comparable with patients treated with the PEG-IFN alpha -2b/RBV combination (3%) and standard IFN/RBV (1%).
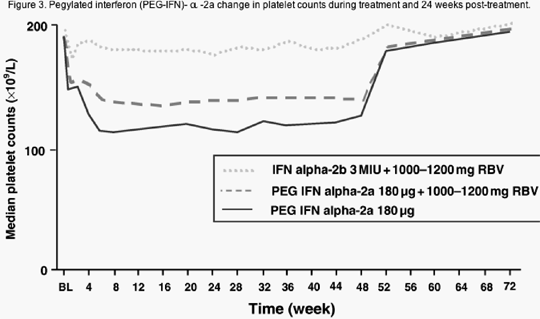
In general, thrombocytopenia is more severe in patients receiving PEG-IFN/RBV than in those receiving IFN alpha -2b/RBV, but not as severe in those who receive PEG-IFN therapy alone, 3 suggesting that some reactive thrombocytosis may be occurring due to RBV-induced anaemia (Figure 3).
|
|
| |
| |
 |
|
| |
| |
Dose reduction is advised when platelet counts fall below 50 000/ mu L. Although discontinuation of therapy is usually unnecessary, it would be recommended if platelet counts fell below 30 000/ mu L. The use of IL-11 (Oprelvekin) has been reported in a small pilot trial for treatment of HCV treatment-related thrombocytopenia. Although the compound did improve platelet counts by week 24, this was associated with lower extremity oedema and is currently not recommended. No adverse events conclusively related to spontaneous internal haemorrhage because of treatment-related thrombocytopenia have been reported to date.
Anaemia
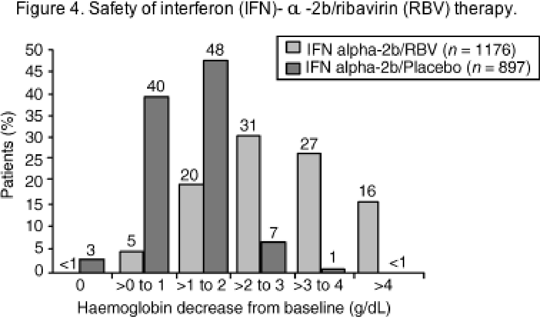
As shown in Figure 4, most patients treated with IFN/RBV combination therapy experience anaemia of grade 1-2 severity (mild-to-moderate). The anaemia is probably multifactorial in most cases. Some degree of haemolysis is virtually universal in patients receiving RBV and plasma levels correlate closely with the degree of anaemia. Erythrocytes selectively accumulate RBV metabolites, sustain oxidative membrane damage and become subject to increased extravascular haemolysis in the reticuloendothelial system. In addition, the IFNs can directly suppress bone marrow erythropoiesis, thus impairing the response to RBV-induced haemolysis. Ribavirin itself has also been reported to have myelosuppressive properties.
|
|
| |
| |
 |
|
| |
| |
The conventional management of anaemia during hepatitis C combination therapy, and that recommended by the drug manufacturers, is to reduce the RBV dose for haemoglobin (Hb) levels of <10 g/dL and obtain blood count levels every 2 weeks or more frequently, if indicated. Individuals with cardiac disease may require early discontinuation of RBV if they become anaemic. In all patients, RBV is recommended to be discontinued if the Hb falls below 8.5 g/dL.
In a large study population treated with combination PEG-IFN alpha -2a and RBV, some 19-22% of patients required reduction of RBV dose as a result of anaemia during therapy. This was a higher proportion of patients requiring dose reduction than in the trial of PEG-IFN alpha -2b/RBV (12-13%) and most likely reflects the lower dose of RBV used in that study. Weight-based RBV dosing at 1000-1200 mg/day is mandatory for patients infected with genotype 1 HCV, whereas a lower dose of RBV (800 mg/kg/day) is acceptable in those with genotypes 2 or 3.
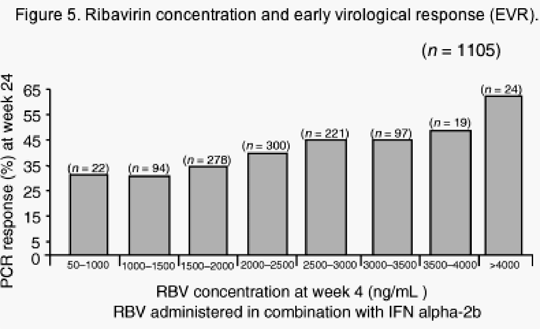
As there appears to be relationship between RBV dosage and SVR rate, 2 it is preferable not to dose reduce RBV where possible, particularly in patients with genotype 1. Figure 5 shows the relationship between RBV concentration in red blood cells at week 4 and HCV RNA polymerase chain reaction (PCR) response at week 24, suggesting that the more RBV present, the better the anti-viral response.
|
|
| |
| |
 |
|
| |
| |
A regression analysis of response rates and RBV dosage revealed a clear direct relationship between SVR and the administered dose, with a definitive cutoff at a critical dose of 10.6 mg/kg (see Figure 6). 2 This retrospective analysis argued that strict adherence to RBV dosage improved SVR rate, although this remains unproven in a prospective fashion.
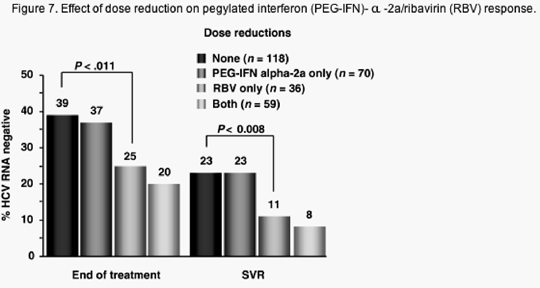
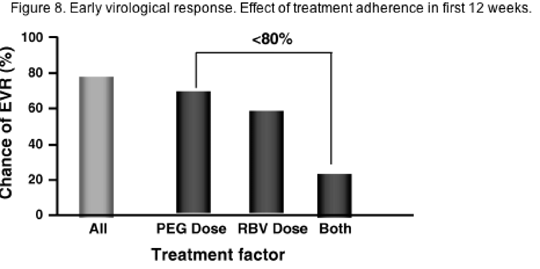
Data from the lead-in phase of the HALT-C trial indicates that in retreatment of prior IFN or IFN/RBV non-responders, adherence to RBV dosage is critical during the first 12 weeks of therapy but not so crucial after 12-20 weeks. Surprisingly, adherence to RBV dosage seemed more important in this population than adherence to PEG-IFN alpha -2a, where dose reduction seemed to have little effect on SVR (see Figure 7). A recent re-analysis of data published on PEG-IFN alpha -2b/RBV has also indicated that dose reduction of RBV during the first 12 weeks of treatment had a greater effect on early virological response (EVR) than dose reduction of PEG-IFN alpha -2a, although the number of patients analysed was small (Figure 8).
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
The trial by Fried et al. demonstrated that full dose scheduling of both PEG-IFN and RBV permits 75% of early virological responders to have an ultimate SVR. Dose reduction resulted in a fall to 67% in those individuals, but discontinuation resulted in a loss of SVR in all but 12%. Taken together, these data suggest that dose reduction is certainly preferable to discontinuation of RBV, but also emphasize that adherence to RBV dosage during the first 12 weeks of treatment is critical to ultimately obtaining an SVR. Hence, any measures that reduce the haematological AEs of RBV and keep patients on full therapy are likely to be of benefit.
In response to the haemolytic process, patients who develop anaemia on RBV therapy usually have a reactive reticulocytosis. However, De Franceschi et al. have shown that the compensatory reticulocytosis in patients treated with combination therapy is much less than that seen in patients treated with RBV alone. The use of Epoetin alpha (erythropoietin) has therefore been studied to see if it can correct the inadequate haemopoiesis and improve the anaemia in patients treated with combination therapy.
A recent randomized, prospective, double-blind, placebo-controlled trial demonstrated that Epoetin alpha given at 40 000-60 000 international units weekly significantly improved Hb levels in patients receiving combination therapy. 49 Approximately 88% of the patients receiving Epoetin alpha were able to maintain their initial dose of RBV as opposed to 60% of patients receiving placebo (P <= 0.001). The mean RBV dosage maintained on treatment significantly improved within the first 8 weeks of therapy in patients receiving Epoetin alpha and Hb levels improved from 10.6 ± 0.9 to 13.2 ± 1.2 g/dL in those patients receiving Epoetin (P <= 0.0001). There was also a highly significant improvement in overall QoL in patients receiving Epoetin in the first 8 weeks of therapy, with increased energy and activity levels in particular.
It is hoped that these data will translate into improved compliance for these patients and thereby ultimately improve their SVR rate, although this is currently still conjectural. The safety profile of Epoetin alpha in this trial revealed that the majority of patients tolerated the drug without adverse events. Therefore, it appears to be an acceptable method of maintaining RBV dosage and improving QoL. However, the cost of Epoetin alpha is substantial and must be considered in making these decisions.
Epoietin alpha therapy may also be of use in aiding recovery from anaemia in patients given combination anti-viral treatment. The return of Hb levels to baseline may take longer in subjects who have been treated with PEG-IFN/RBV than reported for patients treated with standard IFN/RBV. Furthermore, in patients treated with PEG-IFN and RBV, return to baseline Hb levels does not necessarily occur in all patients by follow-up weeks 12 or 24. Theoretically at least, erythopoietin use may facilitate a more rapid recovery from anaemia and further studies on its use after treatment with PEG-IFN/RBV are warranted.
Data has now been presented (but not published) indicating a similar response to Darbepoetin alfa in an open-label pilot study of HCV patients treated with PEG-IFN and RBV. The interim analysis of this trial suggests that patients with RBV-induced anaemia are able to achieve and maintain a Hb level >10.2 and <12.0 g/dL when given 3.0 mcg/kg of Darbepoetin every 2 weeks. Patients were able to maintain the initial RBV dosage 92% of the time and had a significant improvement in QoL as seen in the Epoetin alfa trial.
In the future, it may be possible to use anti-viral agents that have less haematological side-effects. When considering analogues which could have the beneficial effects of RBV without the adverse events, two key observations have been made that are critical to drug development: (i) that RBV monotherapy is not effective at inducing a sustained virological clearance, and (ii) that the haemolytic anaemia caused by RBV has limited its dosing.
The exact mechanism of RBV's therapeutic action is still debated, although four proposed models (not necessarily mutually exclusive) have been described. Therefore, one would surmise that any drug that is intended to replace RBV will need to mimic one or more of these proposed mechanisms of action. As mentioned above, the avid uptake and phosphorylation of RBV in erythrocytes appear responsible for the profound haemolytic anaemia in a dose-dependent manner. In order to avoid this unwanted effect, novel compounds have been designed that are either not taken up or phosphorylated by red cells or, instead, are more avidly taken up and phosphorylated in the liver. Both approaches have potential drawbacks, as they could eliminate key steps responsible for the therapeutic success of RBV.
One such compound is levovirin, a nucleoside analogue that is the l-enantiomer of RBV with similar immunomodulatory actions but no direct anti-viral activity in vitro. Unlike RBV, the drug is not recognized by the host kinase enzyme, is therefore not phosphorylated and does not accumulate in erythrocytes. Therefore, haemolytic anaemia does not appear to occur in either normal or HCV-infected patients given levovirin.
A second RBV analogue currently under study is viramidine, the amidine inversion of RBV. This prodrug is rapidly converted into RBV by the enzyme adenosine deaminase, as the liver is exposed to the first pass following an oral dose, which favours the accumulation of RBV in hepatocytes rather than the rest of the body and avoids erythrocytes in particular.
The results of a phase 2 multicentre trial studying three doses of viramidine at 400, 600, and 800 mg b.d., respectively, vs. RBV 600 mg b.d. for a 24-week period have recently been presented, although not published at the time of writing this review. Viramidine had a similar efficacy to RBV at all dosages with no patients developing anaemia (defined as a Hb below 10 g/dL) on the two lower doses of viramidine and only 7% on the highest. The 600 mg b.d. dosage was felt to have the best ratio of efficacy to AEs and this drug will be further evaluated in phase 3 clinical trials.
Conclusions
The side-effect profile of our current regimen of treatment for chronic hepatitis C is potentially severe, dose limiting and a barrier to therapy for many patients and their doctors. As such, the management of the side-effects caused by combination PEG-IFN and RBV therapy is critical to a successful outcome. Recent work has shown that adherence to therapy, particularly in the first 12 weeks of treatment, is essential in order to achieve an EVR and this in turn is vital for attaining an SVR. We have also learned that dose reduction after this critical period, both of PEG-IFN and/or RBV, may not be as critical as during the initial treatment period. Dose reduction of either compound appears to have much smaller effects on SVR than early termination of therapy. We now have available to us a wide range of pharmaceuticals which are helpful in the management of treatment related side-effects including antidepressants, analgesics and haematological growth factors. Cautious use of those agents combined with careful attention to the patient's needs have resulted in improved adherence to therapy and should, ultimately, improve rates of viral eradication. Future management is likely to centre on developing therapies with fewer AEs.
|
|
| |
| |
|
|
|