| |
Analyzing Sleep Abnormalities in HIV-Infected Patients Treated with Efavirenz
|
| |
| |
"Efavirenz increases sleep latency and decreases sleep duration"
By Will Boggs, MD
NEW YORK (Reuters Health) - Efavirenz induces a variety of sleep abnormalities, especially at higher plasma concentrations, according to a report in the February 1st issue of Clinical Infectious Diseases.
Efavirenz therapy has been associated with vivid dreams, difficulty falling asleep, and night awakenings, the authors report, but less is known about whether efavirenz induces abnormalities in sleep architecture.
Dr. Vincent Soriano from Instituto de Salud Carlos III, Madrid, Spain and colleagues performed ambulatory electroencephalogram monitoring to assess the sleep of 18 HIV-infected subjects treated with efavirenz (13 with insomnia, 5 without) and of 13 healthy controls.
Patients with efavirenz-associated insomnia had low sleep efficiency values, increased total time awake and compensatory napping, and a greater number of night arousals, the authors report.
Efavirenz-treated patients, whether or not they reported insomnia, had increased sleep latency, the report indicates, as well as significantly reduced proportions of nonrapid eye movement stages 3 and 4 (deep sleep).
HIV-infected patients with insomnia showed higher concentrations of efavirenz, though not statistically significant, the researchers note, and sleep efficiencies below 90% were nearly twice as common among patients with plasma efavirenz concentrations above 4 mg/mL than among patients whose concentrations were lower.
"Some toxicities in patients on efavirenz, as insomnia, seem to be dose-related," Dr. Soriano told Reuters Health. "Therefore, determining efavirenz plasma levels may allow [the physician] to adjust doses."
Dr. Soriano said that the mechanism behind efavirenz-associated insomnia is "unclear so far. Direct inhibition of some serotonergic pathways in the hypothalamus by efavirenz seems to be the most likely mechanism."
Clin Infect Dis 2004;38:430-432.
Full Text of Article
Analyzing Sleep Abnormalities in HIV-Infected Patients Treated with Efavirenz
Lucía Gallego,1 Pablo Barreiro,1 Rafael del Río,3 Daniel González de Requena,2 Apolinar Rodríguez-Albariño,3 Juan González-Lahoz,1 and Vincent Soriano1
1Service of Infectious Diseases and 2Service of Pharmacy, Hospital Carlos III, Instituto de Salud Carlos III, and 3Service of Clinical Neurophysiology, Hospital La Paz, Madrid, Spain
ABSTRACT
Ambulatory electroencephalogram monitoring was performed for 18 HIV-infected subjects treated with efavirenz with and without insomnia and for 13 healthy control subjects. All patients receiving efavirenz had longer sleep latencies and shorter duration of deep sleep, although poor sleepers also showed reduced sleep efficiency and shorter duration of rapid eye movement sleep. Efavirenz plasma levels were higher in patients with insomnia and/or reduced sleep efficiency.
Sleep disorders are common in HIV-infected persons. Although HIV infection per se has been implied in causing sleep abnormalities, these abnormalities are more frequent in subjects receiving efavirenz (Efv). Vivid dreams, difficulties in falling asleep, and/or numerous night awakenings are frequently reported by patients after beginning Efv therapy. Although these symptoms tend to resolve within the first weeks of therapy, they persist in a significant proportion of patients, causing measurable quality of life impairment and occasionally leading to Efv withdrawal.
It is not well known whether Efv-related insomnia is characterized only by a reduction in time sleeping, or whether more-profound abnormalities in sleep architecture are produced. To answer this question, we studied electroencephalographic (EEG) activity in HIV-infected patients receiving Efv therapy. The presence of insomnia and the potential relationship between insomnia and Efv treatment were established through psychiatric interviews.
Since preliminary data have suggested that sleep alterations might be more frequent in HIV-infected patients with higher plasma Efv levels, we decided, in our study, to examine the clinical diagnosis of insomnia and the EEG findings with respect to plasma Efv levels.
Patients and methods. A total of 26 HIV-infected patients receiving a triple antiretroviral combination including Efv (600 mg once a day at bedtime) for >12 weeks were selected in a prospective and nonrandomized fashion. A semistructured psychiatric interview was performed to rule out any history of mental disordersincluding sleep alterationsand of consumption of licit or illicit psychoactive substances. A total of 8 patients were excluded after this clinical evaluation; 1 had a history of severe psychiatric morbidity, 3 had chronic sleep abnormalities, 2 were long-term users of hypnotics, and 2 admitted current substance abuse.
The presence of insomnia for the 18 patients enrolled was established through sleep diaries and the Pittsburgh Sleep Quality Index (PSQI). Each day for 1 week, each patient recorded, in sleep diaries, variables such as bedtime, total sleep time, time until sleep onset, number of awakenings, use of sleep medications, morning wake time, and a rating of subjective quality of sleep and daytime symptoms of sleep deprivation (fatigue, irritability, and impaired concentration). The PSQI is a composite score generated from 19 items assessing several sleep-associated characteristics (quality, latency, duration, disturbances, use of hypnotics, nighttime restoration, and daytime symptoms of sleep deprivation). Patients having a final score of 5 were considered poor sleepers. Both questionnaires were administered and interpreted by an experienced psychiatrist.
On the basis of the results of the psychometric tools, Efv-related insomnia was diagnosed in 13 of 18 patients, whereas the remaining 5 subjects had no significant sleep alterations. All 18 HIV-positive patients receiving Efv and 13 healthy HIV-negative control subjects underwent ambulatory EEG monitoring. A system with 8 channels of continuous EEG, electromyogram, and electrooculogram monitoring, and a push-button marker of bedtime and nighttime arousals were used for 24 h. The data registered by this system were interpreted according to current international guidelines by a single experienced neurophysiologist who was blinded to the results of the sleep psychometric tests. Finally, blood samples were obtained from all HIV-infected patients to determine plasma Efv concentrations using high-performance liquid chromatography. The blood samples were obtained in the morning before the patients had eaten breakfast, 12 h after the last Efv dose was taken. The EEG recording was performed thereafter. Drug adherence for all HIV-positive patients was assessed using the validated Simplified Medication Adherence Questionnaire.
Results. Sex distribution (84% male) and mean age ± SD (42 ± 7 years) were comparable between HIV-infected patients and healthy volunteers. One-half of HIV-positive individuals were homosexual men, 5 (28%) were heterosexuals, and 3 (17%) were former injection drug users. At the time of sleep evaluation, the mean CD4 count (±SD) was 420.3 ± 172.4 cells/mm3, the median virus load was 90 HIV-RNA copies/mL (range, <50266,255 copies/mL), and the mean time of exposure to Efv (±SD) was 6 ± 3 months in the HIV-positive population.
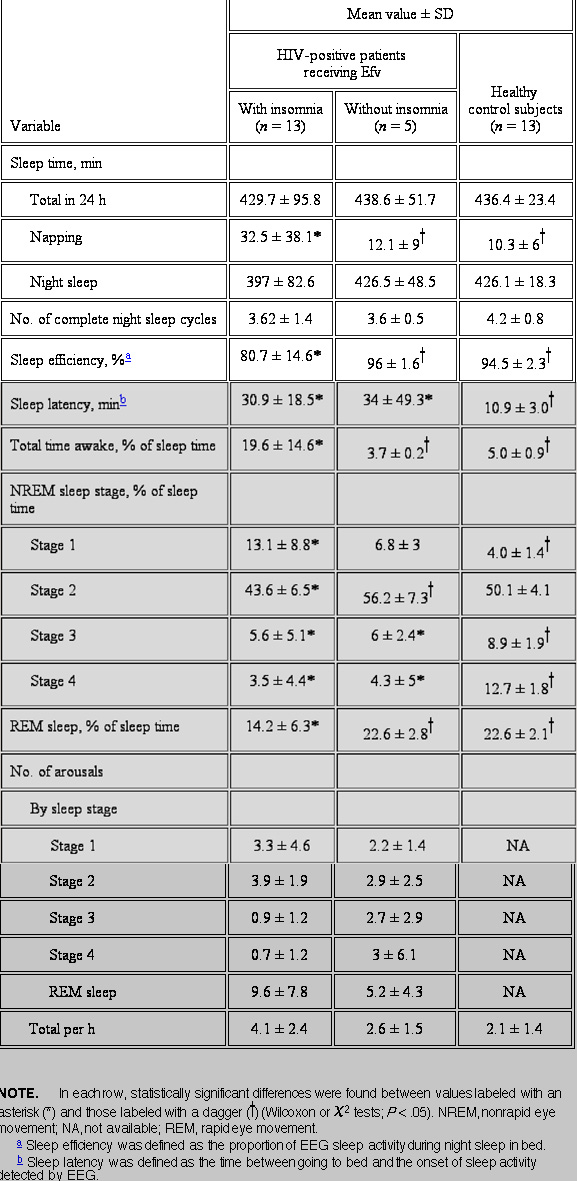
The sleep patterns observed in the 18 HIV-positive patients receiving Efv and in the 13 healthy control subjects are summarized in table 1. Patients with Efv-related insomnia showed low values of sleep efficiency (mean, 81%), whereas this parameter which reflects the proportion of sleep during the night remained within normal limits (i.e., >90%) both in HIV-positive patients without insomnia and in healthy control subjects (P < .05). The total time awake was significantly longer in poor sleepers receiving Efv, mainly due to the increase in the number of night arousals (mean number per night, 4.1 vs. 2.6). A significant increase in sleep latency, which implies difficulties in falling asleep, was noticed among HIV-positive patients receiving Efv, compared with sleep latency in healthy volunteers. Compensatory napping, manifest by EEG sleep activity registered during the day, was more frequent in subjects with Efv-related insomnia, probably in response to the loss of nocturnal sleep restoration.
|
|
| |
| |
 |
|
| |
| |
With respect to sleep patterns, patients with insomnia had less rapid eye movement sleep (REM) than did HIV-positive patients without insomnia and healthy control subjects (mean percentage of sleep time ± SD, 14.2 ± 6.3 vs. 22.6 ± 2.8 and 22.6 ± 2.1%, respectively; P < .05). Moreover, the proportion of nonrapid eye movement (NREM) stages 3 and 4 (deep sleep) was significantly reduced in HIV-infected patients receiving EFV, irrespective of the diagnosis of insomnia, in comparison with that in healthy control subjects (mean percentage of sleep time ± SD, 4 ± 4.2 vs. 10.8 ± 1.8, P < .05).
As sleep fragmentation was one of the most striking findings in our study, the distribution of arousals along the different phases of sleep was analyzed in more detail among HIV-infected patients. Poor sleepers receiving Efv showed a higher number of awakenings during REM sleep than did HIV-positive subjects without insomnia. Conversely, the frequency of arousals was lower during deep-sleep phases in subjects with insomnia, compared with the frequency in normal sleepers. The differences did not reach statistical significance, most likely due to the small size of the study population.
Among HIV-infected individuals, those with insomnia showed higher drug concentrations than did patients with normal sleep (mean ± SD, 4.3 ± 2.9 vs. 2.7 ± 0.7 mg/L, respectively; P value not significant). Moreover, sleep efficiency of <90% was nearly 2 times more common among patients with plasma Efv concentrations of >4 mg/L than among patients with Efv levels below this value (found in 62.5% vs. 37.5% of patients, respectively; P = .04).
Discussion. This study explored the underlying alterations in EEG patterns among HIV-positive patients with Efv-related insomnia. The selected cohort had no history of insomnia or other mental disorders, and was not receiving substances with potential for sleep interference, such as psychoactive drugs, steroids, decongestants, or beta blockers. Heavy alcohol or caffeine consumption was also ruled out in a psychiatric evaluation. Thus, the introduction of Efv was the only recognizable cause of the observed sleep alterations.
EEG records showed that patients with Efv-related insomnia presented a remarkable reduction in sleep efficiency, which seemed to be due to difficulties in falling asleep and to a higher number of sleep interruptions. Regarding sleep architecture, the reduction in the duration of REM sleep was frequent among poor sleepers. The suppression of this stage of sleep can result in an increased intensity of the remaining REM episodes, a phenomenon that has been related to the development of vivid dreams and that is often reported by patients receiving Efv. The enhancement of dreaming activity could precipitate the occurrence of premature arousals, as shown by the fact that sleep interruptions took place during REM sleep stages in 52% of poor sleepers receiving Efv, but in only 32% of HIV-positive subjects without insomnia.
A significant reduction in the duration of deep sleep was noticed in HIV-infected individuals, both in normal and poor sleepers, in comparison with healthy volunteers. These phases of sleep appear to be involved in physical restoration, and reduction in the amount of deep sleep may result in fatigue, somnolence, and distractibility during the day.
In conclusion, Efv-related neurologic adverse effects (especially insomnia, but also other neuropsychological symptoms) may be explained by alterations in sleep architecture. EEG monitoring may be a helpful tool to detect objective sleep abnormalities in patients complaining of insomnia while receiving Efv, especially for those with few therapeutic alternatives to Efv. The observance that plasma Efv levels correlate with reductions in sleep efficiency opens a door to investigate whether adjusting Efv dosages could ameliorate sleep disturbances without compromising the drug's virologic efficacy.
|
|
| |
| |
|
|
|