| |
Report 2 on Sculptra (Newfill): FDA study review; advocacy issues
|
| |
| |
Reported by Jules Levin
On March 25, the FDA convened a panel to review study results and public testimony from patients regarding an application by the company, Aventis/Dermik, for accelerated approval of Sculptra (Newfill). This a cosmetic facial procedure for HIV-related lipoatrophy (fat loss) in the face. The FDA panel voted to 9-0 to recommend that FDA grant conditional approval for correction of facial shape and contour deficiencies resulting from facial fat loss associated with HAART for HIV. However, the FDA has not yet approved Newfill. Usually, when an FDA Panel recommends approval, the FDA follows with approval. In this case, there are some concerns regarding training of doctors, pricing, access to treatment, reimbursement. This might be a good time for community advocates to consider approaching the FDA and the company to address these issues. Perhaps Dermik can be held to have certain standards as more traditional pharmaceutical companies do regarding HIV drugs.
ADVOCACY ISSUES:
(1) ACCESS & PRICE. The Newfill procedure is expensive and calls for repeat injections. This raises the issue of access and price. The company, Aventis/Dermik, should be approached about pricing policies, as standard pharmaceutical companies are approached by community advocacy groups. Pricing can affect access to those with some financial means but what about patients with little financial resources. Should they be denied access? Perhaps, a Patient Assistance Program should be implemented. Should ADAP & Medicaid reimburse for this considering the current tight financial squeeze where life saving drugs such Fuzeon and hepatitis C therapies are not covered; where public reimbursement systems are so strapped that ADAPs are limiting access to HAART and starting waiting lists for patients to access HAART.
(2) DOCTOR TRAINING. The qualifications and training of the doctors implementing Newfill procedures appear to be crucial to safety and efficacy. Aventis/Dermik could be approached directly for negotiations on implementing a standard for quality of care and training; and perhaps an FDA monitored training program for doctors.
Two Sculptra patients testified at the public hearing about the social stigma and depression they experienced with facial lipoatrophy. Apparently, the panelists voted in favor for approval out of compassion for patients, but they were critical of the data from the company. Dermik presented results from 5 studies, all of which were open-label, non-randomized, single-center, investigator-sponsored trials with a total of 400 patients. The data from the studies are reported below, as reported at the hearing by the FDA. The data has gaps if you were to compare this data to that presented to the FDA for HIV drugs. A report on the poster presented at the 11th Retrovirus Conference (Feb 2004) on Newfill is presented below; it discusses repeat injections and concerns about treatment benefits fading and the need for repeated injections. In this poster it was reported that 2% (2 patients) of patients receiving Newfill treatment experienced nerve palsy or anaphylaxis, a serious adverse event and cause for concern.
80% of the study participants were white males. Total skin fat thickness change was the surrogate endpoint for the studies, and the FDA reviewers reported significant enhancement of average dermal thickness among patients treated in the English and French studies. But, some panelists suggested that the evidence would have been inadequate to support the request for market approval if the need were not so great. The results as presented by the FDA from the 5 studies are reported below. No significant adverse events were reported but nodules, site bruising, injection site discomfort, site inflammation, and site erthema were reported. Apparently, no long term safety data were presented. Quality of life assessments showed improvement.
As conditions of approval, the panel recommended FDA require a 2 to 5 year post market study. Future research should examine potential for differences with women, minorities and patients with normal immune systems. The panel urged the FDA to use strong warning in label to prevent Sculptra from being used off label for non-HIV+ patients.
Here is a report written by David Wohl, MD (University of North Carolina) reviewing the poster abstract from the 11th Retrovirus Conference on Newfill study results. Following is the FDA report from the hearing.
Newfill -- Longer Term Safety and Efficacy Data
The select 'before' and 'after' pictures that accompany every presentation regarding Newfill (polylactic acid) are striking and have played no small part in the increasingly fervent drum beat demanding access to the procedure in the U.S. However, despite the desire (and need) for a treatment for disfiguring facial fat loss, there are few published investigations of the safety and efficacy of Newfill even though the compound is available in Europe. A recent article in the journal AIDS (Valantin MA, et al. AIDS. 2003 21;17(17):2471-7) described 50 patients who received Newfill and positive response and minimal adverse effects were reported.
At the 11th Conference on Retroviruses and Opportunistic Infections the authors of previous reports on Newfill provided longer term and more detailed safety and efficacy data than prior presentations (Abstract 726). A cohort of 94 patients receiving intradermal injections of Newfill to the face was followed longitudinally. Subjects had self-described and clinician confirmed lipoatrophy of the face, a CD4+ cell count of >200/uL, a viral load of <20,000 and were on stable ART. Those with bleeding disorders, low platelets (<80,000), or receiving non-steroidal anti-inflammatory medications (ibuprofen, aspirin, etc) were excluded. Subjects were almost all men (94%) and white (93%). Facial lipoatrophy at baseline was judged as "light" in 36%, "moderate" in 43% and "severe" in 21%. Injections of Newfill were given every 15 days. Approximately 3 mL of polylactic acid was injected along with lidocaine. Following injection, the area was iced and then massaged in an attempt to reduce the risk of focal accumulation of the compound.
Efficacy was primarily assessed using an Analogic Visual Scale Satisfaction Index (AVSSI) in which the subject draws a line on a scale of 0-10 (where 0 represents the least and 10 the most) indicating their satisfaction with the effect of Newfill. A median of 5 series of injections was administered. Notably, 41% of subjects also had injections at the temples. The AVSSI at baseline was 3.4 (recall 0 is least satisfied); at the end of treatment the median score was 6.8 and at 7.5 months following the end of treatment it was 7.0. Using any increase from baseline as the criterion for success, 87% of subjects experienced success at the end of treatment. This fell to 73.3% 7.5 months out from end of treatment. The AVSSI did not improve after the first 3-4 injections and despite the improvement in this measure a survey did not reveal enhancement in quality of life. To obtain a more objective assessment in appearance change three observers blinded to the sequence of sophisticated facial surface photographs of subjects taken at baseline, during and after therapy with Newfill were asked to order the images chronologically. This was correctly accomplished in just under 60% of the cases. An example of three images from a subject who bore an incredible resemblance to U.S. Senate Minority Leader Tom Daschle demonstrated to me the difficulty of the observers' task. The significance of the lower rate of success in this objective assessment versus self-report is likely moot as the most critical determinant of success is how the patient feels he/she looks.
Unfortunately, the probability that a repeat injection was needed during the 15 months following the last Newfill treatment was 43% - indicating the benefits of Newfill diminish with time.
Adverse events were not rare. All patients had some degree of post-injection edema, 77% experienced pain during the procedure (of these 28% required pain medication), 13% had post injection non-inflammatory nodules, 7.5% had vagal hypertonia during the injection which I think means they felt faint, 4% had bruising or bleeding, 1% had inflammatory nodule development, 1% experienced facial palsy of unreported duration ostensibly from the hitting of the facial nerve during treatment and 1% had an anaphylactic reaction (i.e. a very severe allergic reaction). Although, nerve palsy or anaphylaxis occurred in only 2 patients, a 2% rate of very serious adverse events is cause for concern.
Overall, these data may temper early expectations and praise for this procedure and may present some potential hurdles to eventual approval in the U.S. That repeated series of injections of this expensive agent are likely to be required and serious adverse events are to be risked may tilt the risk to benefit ratio for some. For others, the promise of improved appearance supercedes the risk of adverse events. This is understandable and not unique to those living with HIV - Botox is widely popular despite a 3.2% risk of causing eyelid droop. At present a perfect therapy for facial fat wasting does not exist and calls for prevention are meaningless to those already suffering from disfiguring facial fat loss. For these people, especially those living in areas where Newfill is available and who can afford it, this study provides import safety and efficacy data. Data they will need when deciding to inject or not to inject.
Herbert Lerner, MD
Division of General, Restorative and Neurological Devices
Plastic and Reconstructive Surgical Devices Branch
FDA
March 25, 2004
SCULPTRA
New-Fill is the name of the device as it is commercially available outside the US. Sculptra is the intended name of the device as it will be marketed in the US. For this review, the use of these names is interchangeable. The components of the two are identical.
Sculptra- Indication for Use
Sculptra is intended to correct shape and contour deficiencies resulting from facial fat loss (lipoatrophy) in people with human immunodeficiency virus.
Material
Sculptra is a sterile solution consisting of:
--PLLA (poly-L-lactic acid)
--Sodium Carboxy-methyl cellulose
--Mannitol (a alcohol sugar)
--Sterile Water
SCULPTRA
P030050
FDA Review Team
Herb Lerner, MD- Clinical & Lead
Charles Durfor, PhD- Pre-clinical
David Berkowitz, VMD- Toxicology
Phyllis Silverman, MS- Statistics
Kim Struble- PharmD- Clinical (CDER)
Sybil Wellstood- Manufacturing
Mary Wollerton- Patient Labeling
CONTENTS
--Toxicology
--Sculptra Studies
Sculptra Toxicology
Previous Medical uses of Sculptra Components
Toxicology Testing
Hemocompatibility
Results/Comments-Complement activation not affected. Measured CH50 and SC5b-9. Normal hemolysis testing not needed.
Genotoxicity
Results/Comments
No significant mutagenesis. Bacterial reverse mutation assay.
No increase in aberrations. Chromosomal aberration assay.
No increase in micronuclei. Mouse micronucleus Assay
Subchronic Toxicity
Results/Comments
No Toxicity. Intracutaneous 90-days. Normal foreign body reaction to implant material (only 5 sites examined).
Systemic Toxicity
Results/Comments
No Toxicity. Intraperitoneal injections in mice at IP at 5.6 ml/kg.
Sensitization
Results/Comments
No significant sensitization. Sixteen guinea pigs injected intradermally.
Cytotoxicity
Results/Comments
No cytotoxicity. Test article placed directly on agar surface.
Sculptra Physical Characteristics
--Molecular weight 40 - 50 thousand
--PLLA particles irregular shape
--40-63 microns ±
--2 hours required for optimal suspension
--Physically chemically and microbiologically stable for 72 hours after suspended.
Sculptra Resorption Kinetics
--No weight loss for 24 weeks in phosphate buffer at pH 7.4 at 37 degrees C.
--19% weight loss at 50 degrees C.
--Foreign material seen histologically after intradermal implantation for 90 days in rats.
Published In-Vivo Resorption Studies on PLLA
Resorption rate is function of molecular weight, crystallinity, and particle size.
Compact PLLA rods of 95,000 Daltons were implanted subcutaneously in rats.
--1 month 19% degraded
--3 months 40% degraded
--6 months 56% degraded
SCULPTRA STUDY DESIGN
Presented are 5 investigator-sponsored studies.
--2 studies are from Europe
--3 studies are from the US
--All were single center studies
--No study was a randomized, controlled, or blinded study as we are used to seeing for a PMA
--All were Open label
--TCT (Total Cutaneous Thickness)
Vega Study-France
Inclusion Criteria:
HIV+
Plasma HIV viral load <5000 copies/ml
Current HAART treatment >=3 months
HAART for at least 3 years
Buccal adipose tissue <2mm
Exclusion Criteria:
Cutaneous Kaposi's Sarcoma of the face
Infection or concurrent herpes labialis
Previous facial fillers within 6 months
Unwilling to meet study follow-up time tables.
Study Design
Fifty (50) patients enrolled to study effects if the device over time
--47 patients completed the trial, 2 withdrew at 72 weeks (schedules) and 1 withdrew due to an unrelated event.
--Open label, non-randomized, uncontrolled
--Patients were given bi-weekly injections
--Safety endpoints designed to look for changes in biological parameters and AE's
--Efficacy endpoints: change in TCT
Demographics:
Age- (mean) 44.9 ± 6.8
Gender- 98% male
Race-
84% Caucasian
6% Hispanic
4% North African
2% Black African
4% Carribean
AIDS defining event- 50%
CD4 count- 397.1± 168
HIV viral load-(median)- 200 copies/ml (50-96k)
(viral load <5000 copies/ml- 86% of pts.)
TCT cheeks- mean 3.0mm
Adipose tissue <2mm
Endpoints:
Safety- adverse events
--Treatment-related events
--Local and systemic
--Change from baseline CD4 cells
--Viral load
--Blood Lactic Acid levels
Results
Bruising- 3%
Hematoma- 30%
Nodule- 52%
Efficacy- Change from baseline in TCT (mm)
Study demonstrated statistically significant increases from baseline to week 96-
--At 8 weeks mean change 5.2 mm (SD 1.7)
--At 24 weeks change was 6.4 mm (SD 1.6)
--At 48 weeks, change was 7.2mm (SD 1.3)
--At 72 weeks, change was 7.2 mm (SD 1.3)
--At 96 weeks, change was 7.0mm (SD 1.4)
--Photographic Assessment
--Visual Analogue Scale 0-10 scale (with 10 the most satisfying physical/emotional state)
Chelsea & Westminster - England
Inclusion Criteria:
--HIV+
--Moderate to severe lipoatrophy
--Not pregnant or lactating
Exclusion Criteria
--Active opportunistic disease or wasting
--Current growth hormone therapy
--Current chemotherapy for malignancy
--Known hypersensitivity to PLLA
Study Design
--30 patients
--Half of group delayed 12 weeks as a comparator
--30 pts. Treated
--29 pts. Reported (1 declined data disclosure)
--Two groups of 15, the second group had injections delayed for 12 weeks
--Clinical exam, serum CD4 and viral loads obtained
--Facial Ultrasound
--VAS and HAD (Anxiety/ Depression scores)
Demographics:
|
Age- | 41 years (mean) |
|
Gender- | 28 males/ 2 females |
|
Race- |
|
|
72% Caucasian |
|
|
3% Black |
|
|
24% Hispanic |
|
Mead duration of HAART- 5.1 years
Mean baseline CD4 count- 473.6
Viral load (median) - 72.0 copies/ml
Endpoints:
Safety
--Change in viral load
--Change in CD4 count
--Change in blood chemistry
--Adverse Events
Efficacy
--Buccal skin thickness measurements
--Change in facial appearance- MD and Pt. assessments
RESULTS
Adverse events-combined groups
--Injection site bruising- 38%
--Injection site discomfort- 10%
--Injection site erythema- 10%
--Injection site inflammation- 10%
--Injection site nodule- 31%
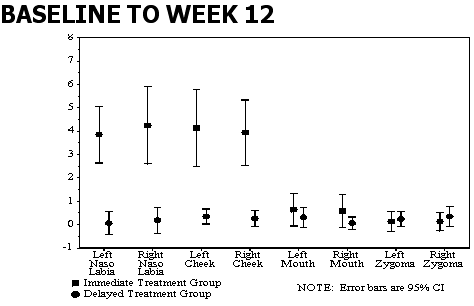
VAS scores improved
Clinical lab parameters unchanged
|
|
| |
| |
 |
|
| |
| |
Common Finding In Both Studies-
Nodule at injection site
--52% VEGA
--31% C&W
Discussion
--Onset average up to 218 days (9 to 748)
--Most reported as mild and not visible
--No histological data available
US Studies
APEX-001
--Investigator Sponsored Compassionate Use Study
--Open label, uncontrolled, non-randomized study
--100 patients
--1-6 treatment sessions (average-3)
--1-8 cc of New-Fill per treatment session
Demographics similar to previous studies
--HIV+ 14 years
--Mean age 44.5
--82/96 patients Caucasian
Inclusion Criteria
--HIV+
--Demonstrable photographic lipoatrophy
Exclusion Criteria
--Active Infection, Kaposi's sarcoma or Herpes on the face
--Facial injections within last 3 months
--Treatment with interferon or steroids
Safety results
Adverse events considered mild
--6 nodules reported in 85 patients at 3 weeks
--39 nodules in 70 patients seen at 12 months
Efficacy results
--High patient satisfaction- 8.8/10
--High investigator rating -- from 3.2 to 1.36 (lower score is better)
APEX002
--Investigator sponsored IDE
--HIV+
--100 patients
--Average of 3.5 treatments/patient
--Similar demographics
--Average time HIV+ 11.9 yrs
--Average years HAART therapy- 13
Adverse Events- mild
Soreness and nodules
--6 nodules in 99 patients
--19 pts. With injection site soreness
--2 pts. With transient fever
High patient satisfaction-
--Scores went from 3.71 to under 1 (lower score is better)
US Study- Hermosa Beach
--Open label, uncontrolled, non-randomized
--Similar demographics and treatment schedule
--1-6 treatments/patient
--Up to 6 cc per treatment
--Average time HIV+ 13 years
--Average time HAART- 9 years
Inclusion/Exclusion criteria
Similar to Apex studies
--HIV+
--Lipoatrophy
--Infections of face, Kaposi's sarcoma
--Treatment with interferon or steroids
--Uncontrolled DM, lactic acidosis
Endpoints
--To evaluate the quantifiable improvement in facial wasting after serial intradermal injections of New-Fill
Safety-
--In repeated treatments
Efficacy
--Durability of New-Fill
--Psychological impact on patients
Results
Adverse events-mild
--8 nodules in 87 patients
High patient satisfaction
Average increase TCT- 6mm @ 6 mos.
--Average mm initial 7.44
--Average mm-end of tx. 13.92
--Average mm- 6 mos. 13.22
--Average mm- increase 5.78
Conclusion- Overall Safety
--In general, the majority of treatment related events are mild pain, bruising and swelling at the injection site.
--Device events are generally palpable subcutaneous nodules (up to 50%)
--No major AE's reported
Conclusion- Overall Efficacy
--TCT analysis in VEGA study demonstrates increased TCT.
--Dermal thickness changes in the C&W study also demonstrate significant enhancement of dermal thickness
--Photographic evidence of sustained efficacy is shown
--Quality of Life assessments show improvement from the baseline
Statistical Summary
--No masking or use of validated severity scale
--Total Skin Thickness (TCT) was used as surrogate endpoint for improved appearance
--Statistically significant (p<0.001) increases in TCT observed in Vega and C&W Studies
--Treatment effect was independent of time on HAART, baseline CD4 counts, or baseline skin thickness
--Increase in skin thickness correlated pictorially with improved appearance
|
|
| |
| |
|
|
|