| |
"The Public Health Impact of Abbott Laboratories' Unreasonable Terms for Norvir" - Community Point of View
|
| |
| |
Presented at a Public Meeting at the National Institutes of Health (NIH) on May 25, 2004
Robert Huff
Editor, GMHC Treatment Issues
Gay Men's Health Crisis, New York
bobhuff@gmhc.org
Good Morning.
My name is Bob Huff. I am the editor of GMHC Treatment Issues, a monthly newsletter about HIV treatment research published by Gay Men's Health Crisis in New York, the world's first and largest AIDS service organization.
We've seen a revolution in AIDS treatments over the past ten years, but the therapies we have are not perfect. I'm here today because I am keenly interested to see that the innovation of more effective and less toxic HIV drugs continues.
In the first part of December 2003, the HIV/AIDS treatment community was shocked to hear that Abbott Laboratories was raising the price of its HIV drug, Norvir, five-fold. The price per 100mg pill would increase from $2.14 to $10.71 (average wholesale prices).
As you've heard, although Norvir was developed and approved by the FDA as an anti-viral drug -- an inhibitor of the HIV protease enzyme -- due to excessive toxicity, it is no longer used as such. Instead it is now used for an off-label indication in much lower doses to take advantage of one of its side effects, namely the inhibition of a metabolic pathway in the liver that effectively improves the concentration of other drugs in the blood. In current clinical practice, most other HIV protease inhibitors are "boosted" by Norvir, which increases their effectiveness. In other words, Norvir enables other drugs to work better.
|
|
| |
| |
 |
|
| |
| |
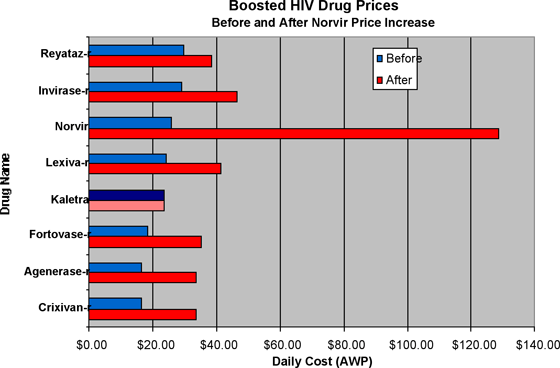
Here is a before-and-after price chart that shows the six approved HIV drugs that can be boosted by Norvir, and how the price increase has affected their overall cost. Note that the price of Norvir in its approved dosage as an antiviral is far out of proportion to the others. Also note that the price of the drug Kaletra, which is also made by Abbott and contains a small boosting dose of Norvir in each pill, did not change and is now the lowest price boosted protease inhibitor on the market. It is clear that the practical and intended effect of the Norvir price increase was to position Kaletra in advantage to its competitors.
|
|
| |
| |
 |
|
| |
| |
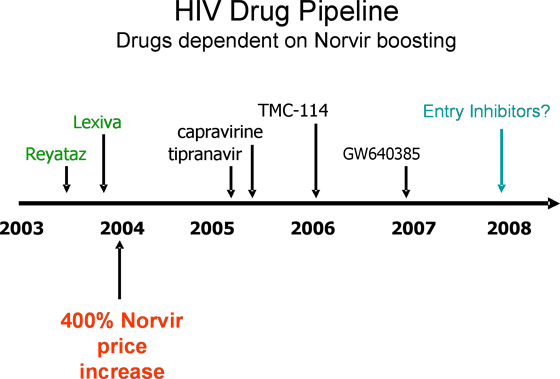
Here is another chart that shows a timeline for the development of some HIV drugs that require Norvir boosting. It includes two protease inhibitors that were approved last year (Reyataz and Lexiva) and several currently in development. It seems clear to me that the Norvir price increase was calculated to come just after these two new drugs received approval.
But I'm more concerned about the drugs that are still on the path to approval -- and about potentially useful drugs that may now never enter clinical development -- because they would be at the mercy of Abbott's monopoly on Norvir.
I would like to argue that Abbott's failure to make Norvir available on reasonable terms will adversely affect the development of new drugs that depend on metabolic boosting and will limit the amount of research that will be conducted on existing drugs that require boosting. I believe that the public health is threatened by the restricted availability of Norvir caused by Abbott's unconscionable price increase.
Abbott's abuse of their patent on Norvir will limit patient access to drugs, limit research, limit options for doctors and limit the innovation of new-generation drugs of this type. This is why you are being asked to protect the public against Abbott's unreasonable use of the Norvir patents.
Before a pharmaceutical manufacturer decides to invest hundreds of millions of dollars into bringing a promising compound along the path to FDA approval, the company projects the market for the drug over the entire expected life of the product. While this isn't easy, given the rapid pace of change in HIV therapy, it is necessary to forecast whether the drug will be competitive and will repay the considerable investment in clinical development. For the makers of Norvir-boosted drugs in the pipeline, Abbott's price increase has thrown these forecasts into chaos.
In seeking to mitigate the impact of the 400% increase in the price of Norvir, Abbott has announced it will make the drug available at the old price for research purposes to companies that are developing a drug that requires Norvir-boosting. However this offer expires once the new Norvir-dependent drug receives FDA approval and goes on the market.
Yet research on these drugs can not and must not end with approval. Post-market research, so-called Phase IV studies, are important to "fill in the blanks" about how a drug behaves in real-world settings and to provide controlled data that helps physicians make the most appropriate use of all the drugs in their armamentarium.
Much of this Phase IV research is mandated by the FDA and some is initiated by the company for marketing purposes. For the recently approved protease inhibitors, the 400% increase in the price of Norvir means that the cost of post-marketing research has now increased dramatically. One pharmaceutical executive estimated that the cost of post-approval research could go up by $20 to $30 million. And this is for drugs that have already been approved, with FDA-mandated post-market research already planned and budgeted.
The impact on drugs still in the pipeline is far more insidious.
A drug company's Phase IV research commitments are decided in negotiations with the FDA. The FDA says it will grant accelerated approval based upon available safety and efficacy data, but only if the company will show a plan for continuing research on the drug after entering the market. These research plans are negotiated based on what the FDA would like to see and what the drug company can afford. The simple fact is that after the 400% rise in the price of Norvir, companies will not be able to afford as much post-market research. And the high price of Norvir will effectively tie the hands of the FDA in what they can ask of companies. This is going to hurt patient care.
There are four Norvir-dependent drugs in the pipeline that this will affect. Abbott's monopoly on Norvir means that there will be less post-marketing research and, consequently, less important real-world medical information produced on how to use these drugs, for example, in women, in people of color, in prisons, in combination with other drugs, in people with hepatitis infections or in people with liver or kidney disease. Much of this research will become too expensive. How much important, useful and desperately needed medical information will never see the light of day because of Abbott's abuse of its patent monopoly on Norvir?
Then there are the government research networks, such as the AIDS Clinical Trials Group (ACTG) at the National Institutes of Health. An investigator might want to use a Norvir-boosted drug in studies of treatment strategies for people with few remaining options, or in women, or in special, under-studied populations. Even if Abbott agrees to provide Norvir for free, these government researchers will have to ask: How useful will the resulting data be down the road if we study drugs that, while promising, will, in practice, be unaffordable and go unused? So, once again, Abbott's Norvir monopoly will hold back research, limit medical knowledge and hurt patient care.
But my main concern is with what Abbott's monopoly on Norvir means for the future. One pharmaceutical executive I spoke to, in evaluating the impact of Abbott's action, posed this as a rhetorical question: "Who would risk developing a Norvir-boosted protease inhibitor after this price increase?" What he meant was that, not only will the high price of Norvir place any new Norvir-dependent drug into an uncompetitive price stratum, but Abbott's unpredictable behavior has made depending on them or their products an unsupportable risk. It's difficult enough to project market conditions for new HIV drugs that don't need Norvir; it's very unlikely that a corporate market analysis will ever again justify investment in drugs of this type. In the words of another pharmaceutical executive, after the drugs currently in the pipeline empty out, "We've seen the end of the line for boosted protease inhibitors."
And that is a shame, because we desperately need new protease inhibitors to treat drug-resistant HIV. The so-called HIV salvage population is the fastest growing market segment in HIV therapy. Drugs with incremental benefits have continued to trickle onto the market over the past few years, but in practice, this has resulted in many patients simply adding the latest therapy onto a failing regimen, which starts the cycle of resistance all over again. Unless a person switches to multiple drugs that his virus is susceptible to, the development of resistance seems inevitable.
For drugs in the protease inhibitor class -- which are very durable HIV therapies -- Norvir has assumed a crucial, enabling role by assuring that sufficient blood levels of the active antiviral drugs are achieved. Looking ahead, we can foresee the continued need for new protease inhibitors that will have novel resistance profiles, that will have less toxicity, and that are more durable. Some of the drugs in the pipeline have some of these qualities, but none has all of them. Most observers expect the protease inhibitors in the pipeline to continue towards approval because their sponsors have already made substantial financial commitments to their development. But how many important, useful, and desperately needed drugs will now never see the light of day -- because of Abbott's monopoly on Norvir? Abbott's unreasonable terms for Norvir will inhibit innovation, restrict research, limit medical options and hurt people with HIV.
Finally, the pricing issue aside, Abbott has not been a responsible custodian of this drug. Although Norvir's usefulness is as a metabolic booster and not as a protease inhibitor as they had hoped, the company has not made the drug available in dosages that would optimize the use of Norvir for this purpose. With only a 100mg pill of Norvir available, many patients who would only require 50mg or less for boosting are being subjected to unnecessary toxicity. (Kurowski)
Furthermore, Abbott has not sought FDA approval for Norvir as a metabolic boosting agent and continues to represent the drug in medically misleading terms -- all the while encouraging continued off-label use.
Also, Abbott has, I have been told by several pharmaceutical executives, been unwilling to offer reasonable terms for licensing Norvir for co-formulation with other companies' drugs, even though a co-formulated pill is widely considered to help simplify drug regimens and improve patient adherence and therapeutic outcomes. The FDA, in a recent guidance document on fixed dose combinations (FDC) said:
"Kaletra (lopinavir/ritonavir), an approved FDC, is an antiretroviral combined with a metabolic booster; a low dose of ritonavir…. Other HIV protease inhibitors are often administered with low doses of ritonavir and may be suitable for co-packaging or co-formulation. FDA encourages sponsors to develop FDCs for this type of drug combination to help in simplifying regimens." (FDA)
Yet Abbott, in order to protect its own, more toxic Kaletra product, continues to resist this.
To sum up, Abbott has behaved unconscionably, and perhaps illegally, in increasing the price of Norvir, and in doing so they have abused the privilege of their patents.
o They have attempted to manipulate the market and restrict patient access to competing drugs that have less toxicity.
o They have increased the financial burden their competitors face in performing important post-market research.
o They have tied the hands of the FDA in how much post-market research can be required of drugs approaching approval.
o They have stifled innovation and have killed the market chances for any new drug candidate that would require Norvir.
o They have not been responsive to the medical need for safer and more rational doses of Norvir.
o They have refused reasonable offers to license Norvir for co-formulation into patient-friendly combinations with other drugs.
With at least ten HIV drugs (and I haven't discussed potential drugs for hepatitis C and other illnesses) dependent on Norvir to achieve optimal efficacy and minimal toxicity, I believe Norvir should be considered a public amenity and be contracted to more responsible custodians.
I'd like to note that I think the case of Norvir is an exceptional one, and that I fully support industry development programs that build on government funded research. It seems clear that the intent of the Bayh-Dole Act was to stimulate innovation, and in this it has been incredibly successful. But it also seems clear that a mechanism was provided to address abuse, and that, in Norvir, we are confronted with that rare case.
Under Abbott's monopoly control of Norvir, drug access (both to Norvir and to dependent drugs), patient care, innovation, research, and medical options are being restricted. The public interest would best be served by making this vital resource more broadly available under much more reasonable terms.
Thank you.
References:
Kurowski ML. Influence of 50 mg, 100 mg and 200 mg ritonavir on the pharmacokinetics of amprenavir after multiple doses in healthy volunteers for once daily and twice daily regimens. 1st IAS Conference on HIV Pathogenesis and Treatment. 8-11 July 2001. Buenos Aires. Abstract 351.
U.S. Food and Drug Administration (FDA). Guidance for Industry: Fixed Dose Combination and Co-Packaged Drug Products for Treatment of HIV. DRAFT GUIDANCE, May 2004.
|
|
| |
| |
|
|
|