| |
Long-Term CD4+ T-Cell Response to Highly Active Antiretroviral Therapy According to Baseline CD4+ T-Cell Count
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 36(2) 1 June 2004
García, Felipe MD, PhD; de Lazzari, Elisa BSc; Plana, Montserrat MD, PhD; Castro, Pedro MD; Mestre, Gabriel MD; Nomdedeu, Meritxell MD; Fumero, Emilio MD; Martínez, Esteban MD, PhD; Mallolas, Josep MD, PhD; Blanco, José L. MD, PhD; Miró, José M. MD, PhD; Pumarola, Tomás MD, PhD; Gallart, Teresa MD; Gatell, José M. MD, PhD
From Clinic Institute of Infectious Diseases and Immunology, IDIBAPS, Hospital Clínic, Faculty of Medicine, University of Barcelona, Spain.
"...In summary, our data confirm that long-term immune recovery is possible regardless baseline CD4+ T-cell count. However, it seems that the median number of CD4 T cells recovered at long term is around 200-300 cells/mm3 and the immune recovery slowed down after approximately 3 years of HAART and reached a plateau after 4-5 years of HAART. Moreover, patients who started therapy with a CD4+ T-cell count <200 cells/mm3 had a higher risk of having a last determination of CD4+ T-cell count low enough to allow the development of some opportunistic infections than patients with a higher level of baseline CD4+ T-cell count..."
AIDS/DEATH EVENTS THAT OCCURRED IN EACH SUBGROUP
|
|
BASELINE CD4 COUNTS
|
|
|
<200
|
200-349
|
350-499
|
>500
|
|
EVENTS
|
|
No
|
362
|
211
|
133
|
88
|
|
Yes
|
46
|
15
|
4
|
2
|
|
TOTAL
|
408
|
226
|
137
|
90
|
Summary
Current treatment guidelines for HIV infection recommend a relatively late initiation of highly active antiretroviral therapy (HAART). Nevertheless, there is still a concern that immune recovery may not be as complete once CD4+ T cells have decreased below a certain threshold.
This study addressed the long-term response of CD4+ T-cell counts in patients on HAART and analyzed the influence of baseline CD4+ T-cell counts, baseline viral load, and age. An observational analysis of evolution of CD4+ T cells in 861 antiretroviral therapy-naive chronic HIV-1-infected patients who started treatment consisting of at least 3 drugs in or after 1996 was performed.
Patients were classified in 4 groups according to baseline CD4+ T cells: <200 cells/mm3, 200-349 cells/mm3, 350-499 cells/mm3, and >=500 cells/mm3. The main outcome measures were proportion of patients with CD4+ T cells <200/mm3 and >500/mm3 at last determination and rate of CD4+ T-cell recovery. Patients were followed-up for a median of 173 weeks (interquartile range [IQR], 100-234). There were no differences in follow-up between the 4 groups.
CD4+ T cells increased in the whole cohort from a median of 214 cells/mm3 (IQR, 90-355) to 499 cells/mm3 (IQR, 312-733) (P < 0.001). Compared with the group with a baseline CD4+ T-cell count of >=500/mm3, the relative risk of having a last determination of CD4+ T-cell counts >200 cells/mm3 was 0.79 (95% CI, 0.75-0.83), 0.92 (95% CI, 0.89-0.96) and 1 for baseline CD4+ T cells <200 cells/mm3, 200-349 cells/mm3, and 350-499 cells/mm3, respectively. The relative risk of having a last determination of CD4+ T-cell counts >500 cells/mm3 was 0.32 (95% CI, 0.27-0.39, P < 0.001), 0.69 (95% CI, 0.60-0.79, P < 0.001), and 0.94 (95% CI, 0.83-1.06, P = 0.38) for baseline CD4+ T-cell counts <200 cells/mm3, 200-349 cells/mm3, and 350-499 cells/mm3, respectively, compared with a baseline CD4+ T-cell count of >=500 cells/mm3.
The increase in CD4+ T cells from baseline was statistically significant and was maintained for up to 4 years of follow-up. This increase seemed to slow down after approximately 3 years and reached a plateau after 4-5 years of follow-up even in patients who achieved and maintained viral suppression in plasma.
Long-term immune recovery is possible regardless of baseline CD4+ T-cell count. However, patients who start therapy with a CD4+ T-cell count <200 cells/mm3 have poorer immunologic outcome as measured by the proportion of patients with CD4+ T cells <200/mm3 or >500/mm3 at last determination. It seems that the immune recovery slows down after approximately 3 years of HAART and reaches a plateau after 4-5 years of HAART.
INTRODUCTION
Several treatment guidelines 1 for HIV infection recommend initiation of antiretroviral therapy in advanced stages of the disease due to the problems of adherence to a sometimes complex therapy, 2 the unlikely possibility of eradication of HIV-1 with highly active antiretroviral therapy (HAART) alone, 3-6 and the risk of drug-related adverse effects, including serious metabolic abnormalities and apparently irreversible fat redistribution syndromes. 7-9 Moreover, there are contradictory data about whether an earlier initiation of therapy (ie, with a CD4+ T-cell count >500/mm3) rather than starting HAART at a later stage 10-12 could offer any significant advantage in terms of further immunologic improvement, development of resistance, control of viral replication by medication, and decreasing rate of clinical events or death.
From recent studies focused on evolution to AIDS and death, 11 it seems prudent to ensure that HAART is initiated before CD4+ T cells drop to <200 cells/mm3. Those studies solely based on a potential response in plasma viral load seem to suggest that antiretroviral therapy could be withheld for indefinite periods. 10 Nevertheless, there is still a concern that immune recovery may not be as complete once CD4+ T cells have been decreased below a certain threshold. 13 In fact, some studies that have evaluated the increase of CD4+ T-cell count mostly in antiretroviral-experienced patients have suggested that, independently from baseline CD4+ T-cell count, CD4+ T cells reach a plateau or slow the rate of immune recovery after 2 years of follow-up. 14-16 However, this does not seem to be the case for patients on HAART whose viral load remains undetectable. 17 Other authors report that the immunologic recovery is associated with age and baseline CD4+ T-cell counts, at least in patients who maintain a good control of viral load with antiretroviral therapy. 16,18 Some of these studies have been performed in small cohorts, with short periods of follow-up, in antiretroviral-experienced patients, or with selected patients having a good response to antiretroviral therapy, limiting the conclusions obtained.
To further define immunologic factors so as to decide when to initiate antiretroviral therapy, we studied the long-term evolution (>5 years) of CD4+ T cells in all antiretroviral-naive patients from our center who started treatment consisting of at least 3 drugs: 2 nucleoside reverse transcriptase inhibitors (NRTIs) + 1 protease inhibitor (PI), 3 NRTIs, or 2 NRTIs + 1 nonnucleoside reverse transcriptase inhibitor (NNRTI) in 1996 or thereafter. Our main interest was to address the following questions: whether there is a point of no return (a CD4 cell count that represents an irreversible biologic threshold beyond which response to therapy is compromised); the amount of CD4+ T cells that could be recovered; the proportion of patients who recovered and maintained the increase of CD4+ T cells for long periods; the rate of recovery during the follow-up; and whether the response depends on the baseline value of CD4+ T cells, baseline viral load, or age.
RESULTS
Long-Term CD4+ T-Cell Response to Highly Active Antiretroviral Therapy
A total of 861 patients were followed up for a median of 173 weeks (IQR, 100-234). CD4+ T cells increased in the whole cohort from a median of 214 cells/mm3 (IQR, 90-355) to 499 cells/mm3 (IQR, 312-733) at last determination (P < 0.001). Patients were classified in 4 groups according to baseline CD4+ T cells: <200 cells/mm3, 200-349 cells/mm3, 350-499 cells/mm3, and >=500 cells/mm3. Patients with CD4+ T-cell counts <200 cells/mm3 at baseline had higher viral load at baseline and were older than the other 3 groups (P < 0.0001, for both comparisons). There were no differences in follow-up between the 4 groups (P = 0.24). The difference between last and baseline CD4+ T-cell counts was lower in patients with a baseline CD4+ T cell >=500/mm3, when it was compared with the remaining 3 groups classified according to baseline CD4+ T cells (P = 0.024). However, we also observed that the proportion of patients with undetectable level of viral load of the group with a baseline CD4+ T cells >=500/mm3 was significantly lower than the proportion observed in the remaining 3 groups classified according to baseline CD4+ T cells (P = 0.001). Some authors suggest that the suppression of viral load influences CD4+ T-cell recovery. Therefore, we studied whether the difference in the increase of CD4+ T-cell counts observed in patients with a baseline CD4+ T cells >=500/mm3, when it was compared with the remaining 3 groups, was explained by the higher proportion of the patients of this group with uncontrolled viral replication. We observed that the differences between last and baseline CD4+ T-cell counts were similar between the groups when the analysis was restricted to patients who maintained an undetectable level of viral load (<200 copies/mL) achieved by 6 months after starting HAART. The median (IQR) differences between last and baseline CD4+ T-cell count in this subgroup of patients (n = 384) were 319 (217-485) cells/mm3 in patients with a baseline CD4+ T cells <200 cells/mm3, 359 (232-522) cells/mm3 in the 200-349 cells/mm3 group, 294 (119-562) cells/mm3 in the 350-499 cells/mm3 group, and 297 (36-464) cells/mm3 in the >=500 cells/mm3 group (comparison among the 4 groups, P = 0.07).
As the duration of time between first and last measurement varies between individuals, we performed a linear regression model to analyze whether the different duration of follow-up between individuals influenced the differences observed between each CD4+ T-cell measurement and baseline one. In this model, the observed difference was the outcome variable, and duration and baseline CD4+ T cells were the covariates. The difference between the last and the first measurement of CD4+ T cells grows constantly as the time between the 2 measurements increases. Furthermore, the increment of CD4+ T cells is lower in the group with baseline CD4+ T-cell counts >500 cells/mm3 than in the group with baseline CD4+ T-cell counts <200 cells/mm3 (data not shown). As we observed above that the viral replication could influence immunologic recovery, we repeated the linear regression model controlling for viral load at last determination. In this model, adjusting for duration, baseline CD4+ T cells, and last measurement of viral load, there were no differences in the increment of CD4+ T cells between the groups of patients classified according to baseline CD4+ T-cell counts. We confirmed that the difference between the last and the first measurement of CD4+ T cells grows constantly as the time between the 2 measurements increases. We also observed that the increment of CD4+ T cells was higher in the group with a last measurement of viral load <200 copies/mL than in the group with a detectable one. The relationship between duration and last viral load does not vary across baseline CD4+ T-cell groups (data not shown), suggesting that the last determination of viral load is an important predictor of CD4 recovery, irrespective of the baseline CD4+ T-cell group.
Twelve percent of patients (n = 101) had a last determination of CD4+ T cells <200 cells/mm3. Fifty percent of patients (n = 430) had a last determination of CD4+ T cells >500 cells/mm3. CD4+ T-cell count at last determination was >200 cells/mm3 in 79% of patients with a baseline CD4+ T cells <200 cells/mm3, in 93% of the 200-349 cells/mm3 group, and in 100% of the 350-499 cells/mm3 group and >=500 cells/mm3 group (comparison among the 4 groups, P < 0.001). Compared with the group with a baseline CD4+ T cells >=500/mm3, the relative risk of having a last determination of CD4+ T-cell counts >200 cells/mm3 was significantly lower in the patients with a baseline CD4+ T-cell count <350 cells/mm3. Given that the duration of time between the last and first determination varied greatly and influenced the difference between the last and the first measurement of CD4+ T cells, a subanalysis was performed with patients with >36 months of follow-up. The proportion of patients with a last determination of CD4+ T-cell count >200 cells/mm3 was similar between the groups, with the exception of the group of patients with a baseline CD4+ T cells <200 cells/mm3. In this group, the proportion of patients with a CD4+ T-cell count >200 cells/mm3 at last determination increased to 88% [relative risk when compared with the group with a baseline CD4+ >=T cells 500/mm3: 0.87 (95% CI, 0.83-0.92)].
CD4+ T-cell count at last determination was >500 cells/mm3 in 28% of patients with a baseline CD4+ T cells <200 cells/mm3, 58% in the 200-349 cells/mm3 group, 80% in the 350-499 cells/mm3 group, and 84% in the >=500 cells/mm3 group (comparison among the 4 groups, P < 0.001). The relative risk of having a last determination of CD4+ T-cell count >500 cells/mm3 was significantly lower in the patients with a baseline CD4+ T cells <350 cells/mm3, compared with baseline CD4+ T cells >=500 cells/mm3. When patients with >36 months of follow-up were analyzed, there were no differences in the proportion of patients with a last determination of CD4+ T-cell counts >500 cells/mm3 between the groups, with the exception of the group of patients with a baseline CD4+ T cells <200 cells/mm3. In this group, the proportion of patients with a CD4+ T-cell count at last determination >500 cells/mm3 increased to 36% [relative risk when compared with the group with a baseline CD4+ T cells >=500/mm3: 0.41 (95% CI, 0.33-0.50)].
CD4+ T-cell count at last determination was >200 cells/mm3 in 90% of patients who were <40 years of age at baseline and in 85% of the group who were >=40 years old (P = 0.06). There was no statistically significant difference in the relative risk of having a last determination of CD4+ T-cell counts >200 cells/mm3 between both groups. The results were similar when patients with >36 months of follow-up were analyzed (data not shown).
CD4+ T-cell count at last determination was >500 cells/mm3 in 55% of those patients <40 years old at baseline, and in 40% of the group that was >=40 years old (P < 0.001). When patients with >36 months of follow-up were analyzed, the results were similar (data not shown).
Long-Term Evolution of CD4+ T Cells
The increase in CD4+ T cells was statistically significant and was maintained for up to 4 years of follow-up, although it seemed to slow down after approximately 3 years of follow-up and reached a plateau after 4-5 years of follow-up. To analyze this increase, a regression model based on fractional polynomial functions of time and adjusted by CD4+ T cells, viral load, and age was estimated. The model adjusted by baseline viral load and CD4+ T cells fit better the data than the models that included only the CD4+ T cells, or only the age, or a combination of 2 of these variables or the 3 at the same time (data not shown), as compared by BIC. We observed that the model predicting the CD4+ T cells, adjusted by CD4+ T cells and viral load at baseline, was a quadratic function of time, with a high increase during the first year; a much lower increase after 2-3 years of follow-up; a plateau after approximately 4 years of follow-up; and a decrease after 5 years. Therefore, the changes (increase/decrease) in CD4+ T cells were not linear; they represented a parabolic curve identified by the estimated model. This decrease, however, has to be taken with caution given that the number of patients followed up for >5 years was low. If we did not include the data after 5 years of follow-up, the model predicting the CD4+ T cell was also a parabolic function of time with a high increase during the first year, a much lower increase after 2-3 years, and a decrease after approximately 4 years of follow-up. Moreover, the model estimated over the data of the first 4 years of follow-up was a square-root function of time, indicating that during the first year the increase rate of CD4+ T cells was rapid and slowed down over the following years, reaching a stabilization of CD4+ T-cell increase (data not shown). These results were confirmed when the analysis was restricted to patients who maintained an undetectable level of viral load (<200 copies/mL) achieved at 6 months after starting HAART (n = 384).
Patients were classified in 4 groups according to baseline CD4+ T cells: <200 cells/mm3, 200-349 cells/mm3, 350-499 cells/mm3, and >=500 cells/mm3 or higher. There were no differences in follow-up between the 4 groups classified according to baseline CD4+ T cells. The increase in CD4+ T cells was statistically significant and was maintained for up to 4 years of follow-up in the 4 groups. In all groups, the increase in CD4+ T cells was rapid during the first year and slowed down over the following years, reaching a plateau after 4-5 years of follow-up. This result was found by estimating a regression model based on fractional polynomial functions of time and adjusted by CD4+ T cells for each group. We observed that the model predicting the CD4+ T-cell increase in the group with a baseline CD4+ T cells <200 cells/mm3 adjusted by CD4+ T cells was a quadratic function of time, a square-root function of time in the group with a baseline CD4+ T cells 200-349 cells/mm3, a linear function of time in the group with a baseline CD4+ T cells 350-499 cells/mm3, and a logarithm function of time in the group with a baseline CD4+ T cells >=500 cells/mm3. In all these models, there was a high increase during the first year, a much lower increase after 2-3 years, and a plateau after 4-5 years of follow-up.
Time to AIDS or Death According to Baseline CD4+ T Cells and Baseline Viral Load
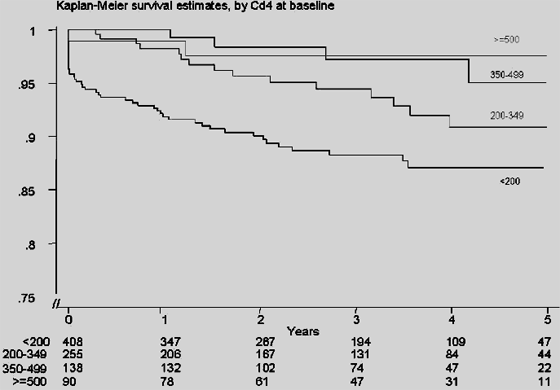
Figure 4 shows the Kaplan-Meier survival estimate of the proportion of patients without progression to AIDS or death of treatment-naive patients starting antiretroviral therapy according to baseline CD4+ T-cell count. A higher proportion of patients with baseline CD4+ T cells <200 cells/mm3 progressed to AIDS (new events after inclusion in the study) or death. There was no apparent difference between the 3 higher CD4 T-cell count categories (global Wilcoxon test for equality of survivor function, P < 0.001).
|
|
| |
| |
 |
|
| |
| |
Kaplan-Meier survival estimate of the proportion of patients without progression to AIDS or death was also calculated according to age. A trend to lower progression was observed in the group with an age at baseline of <40 years old, when compared with the group with age at baseline of >=40 years old (P = 0.07). However, differences in progression to AIDS or death between the 2 age groups were not observed when Kaplan-Meier survival estimate was calculated according to age by controlling for CD4+ T-cell count, suggesting that the effect on progression was really a CD4+ T-cell count effect.
The patients were classified in 3 groups according to baseline viral load: <10,000 copies/mL, 10,000-100,000 copies/mL, and >100,000 copies/mL. There were no differences in time to progression to AIDS or death estimated for the groups identified by the combinations of the category of baseline viral load and CD4+ T cells. Table 4 shows results from the corresponding Cox proportional hazards model before adjustment. With a stepwise selection of the variables with an unadjusted P value of <0.1, only baseline CD4+ T-cell count was found independently associated with AIDS/death over time.
AIDS/DEATH EVENTS THAT OCCURRED IN EACH SUBGROUP
|
|
BASELINE CD4 COUNTS
|
|
|
<200
|
200-349
|
350-499
|
>500
|
|
EVENTS
|
|
No
|
362
|
211
|
133
|
88
|
|
Yes
|
46
|
15
|
4
|
2
|
|
TOTAL
|
408
|
226
|
137
|
90
|
AUTHOR DISCUSSION
Our study suggests that there is not a point of no return when HAART is used in naive patients regardless of baseline CD4+ T-cell counts when the therapy was started. Despite these positive data, and considering the cohort as a whole, CD4 T-cell counts did not recover to a normal value (CD4+ T cells increased from a median of 214 at baseline to 499 cells/mm3 at last determination). This same observation has been reported in a cohort of 314 patients reported by Tarwater et al 14 (CD4+ T cells increased from a median of 249 at baseline to 410 cells/mm3 at last determination). Conversely, CD4 T-cell count recovered to normal value in a cohort of 95 antiretroviral-naive HIV-1-infected patients, 18 who suppressed plasma viral load to level <400 copies/mL with HAART during a median observation period of 45 months. In this last cohort, the median CD4+ T-cell count at baseline was higher than in the other 2 cohorts (from a median of 325 at baseline to 624 cells/mm3 at last determination). The data of all 3 of cohorts seem to suggest that the median number of CD4 T cells recovered at 3-4 years of antiretroviral therapy is around 200-300 cells/mm3.
Some authors have suggested that the relative immune recovery of patients with lower baseline CD4+ T-cell count is higher than in patients with a high level of baseline CD4+ T-cell count. This could be an argument favoring the delay in starting antiretroviral therapy. In fact, Tarwater et al found, in their cohort of mainly pretreated patients, an increase of 213 cells/mm3 in patients with a baseline CD4+ T-cell count <200 cells/mm3 and an increase of only 127 cells/mm3 in patients with a baseline CD4+ T-cell count 500-750 cells/mm3. We also found that the difference between last and baseline CD4+ T-cell counts was lower in patients with a baseline CD4+ T-cell count of >=500 cells/mm3, when it was compared with the remaining 3 groups classified according to baseline CD4+ T cells. However, this lower increase was not observed when it was adjusted by viral load at last determination, suggesting that the significantly higher proportion of patients with detectable viral load at last determination in the group of patients with a baseline CD4+ T-cell count of >=500 cells/mm3 or higher was the cause of the lower increase of CD4+ T cells in this group. Moreover, we observed that the differences between last and baseline CD4+ T-cell counts were similar between the groups when the analysis was restricted to patients who maintained an undetectable level of viral load (<200 copies/mL) achieved by month 6 after starting HAART. These data support the findings of other authors suggesting that the patterns of CD4 cell count increase did not vary in patients who control viremia with HAART despite starting therapy at different CD4+ cell count levels and confirm that the suppression of viral load is the best indicator of CD4+ T-cell recovery.
One of the issues we address in this study is the proportion of patients who have a normal level of CD4+ T-cell count or have CD4+ T cells <200 cells/mm3 (being at risk for opportunistic infections) after a long-term period of HAART. In our cohort, only 50% of patients had a last determination of CD4+ T cells >500 cells/mm3. Twelve percent of patients had a last determination of CD4+ T cells <200 cells/mm3. These data are similar to those reported by Kaufmann et al, who studied the CD4+ T-cell recovery after 4 years of HAART in 2235 patients of the Swiss HIV Cohort and observed that at 4 years, 39% of patients had a CD4+ T-cell count >500 cells/mm3 and 16% had a CD4+ T-cell count <200 cells/mm3. Conversely, the above-mentioned data differ from those reported by the same authors 18 in patients with a good response to antiretroviral therapy, with 75% of patients with CD4+ T-cell count >500 cells/mm3 and only 2% of patients with CD4+ T-cell count <200 cells/mm3. These contradictory findings could support the observations made by Staszewski et al and Moing et al, who suggest that the suppression of viral load is the best indicator of CD4+ T-cell recovery.
It was also interesting to analyze whether the response depended on the baseline value of CD4+ T cells. In our cohort, although the median last determination of CD4+ T cells was 346 cells/mm3 in the group with CD4+ T-cell count <200 cells/mm3 at baseline, 21% of patients did not have a last determination of CD4+ T-cell count >200 cells/mm3. This was true also for 7% of patients with a baseline CD4+ T-cell count between 200-349 cells/mm3. Therefore, in our cohort, patients who started therapy with a CD4+ T-cell count <350 cells/mm3 had a higher risk of having a last determination of CD4+ T-cell count low enough to allow the development of opportunistic infections than patients with a higher level of baseline CD4+ T-cell count. If we focus on a complete recovery of CD4+ T-cell count (defined as a CD4+ T-cell count >500 cells/mm3), a significantly higher proportion of patients with a baseline CD4+ T-cell count >500 cells/mm3 had a complete immune recovery when compared with patients who started therapy with CD4+ T-cell count <350 cells/mm3 at baseline. However, when patients with >36 months of follow-up were analyzed, the likelihoods of having a last determination of CD4+ T-cell count <200 cells/mm3 or >500 cells/mm3 were similar in all the groups, with the exception of the group with a baseline CD4+ T-cell count <200 cells/mm3. These data suggest that patients who eventually initiate HAART with a CD4+ T-cell count in the 200-350 cells/mm3 range could get the same degree of clinically relevant immune restoration as patients with a baseline CD4+ T-cell count >350 cells/mm3.
We also address the rate of immunologic recovery during the follow-up, taking into account all the determinations of each patient. The regression model based on fractional polynomial functions of time and adjusted by CD4+ T cells, viral load, and age at baseline shows that there is a significant increase of CD4+ T cells maintained for long periods (up to 4 years of follow-up), as suggested by other authors in other clinical settings. We also observed that the dynamics of increase change with time, as observed by other authors. There was a high increase during the first year, a much lower increase after 2-3 years, and a plateau after 4-5 years of follow-up. These results were confirmed when the analysis was restricted to patients who maintained an undetectable level of viral load (<200 copies/mL) achieved at 6 months after starting HAART. A very similar trend was observed in the 4 groups classified by CD4+ T cells at baseline. Some authors have suggested that this pattern of recovery is partly due to the fact that some patients have started to reach normal CD4+ T-cell levels. However, in our study, patients starting HAART at low CD4+ T-cell counts appeared to recover CD4+ T cells in a similar pattern to those starting HAART at higher CD4 T-cell counts, arguing against this explanation.
One of the drawbacks of our study is that immune recovery may have been limited by incomplete viral suppression. It can be argued that by changing therapy and obtaining a better viral load control, the immune recovery could further improve. However, our cohort represents what happens in clinical practice. In fact, when a decision to start therapy has to be made, the arguments must be based on clinical practice findings and not on the hope of a complete effectiveness of HAART. Moreover, the studies of long-term evolution of CD4+ T cells of antiretroviral-naive patients who always control viral replication does not address the issue that many subjects with lower baseline CD4+ T-cell counts often are unable to attain or maintain adequate viral suppression on currently available antiretroviral therapy and cannot predict what will be the long-term CD4+ T-cell response of patients in whom a first therapy has failed and has to be changed to a rescue alternative. Nevertheless, the subanalysis performed in patients who maintained an undetectable viral load 6 months after starting HAART confirmed the data of the whole cohort.
In summary, our data confirm that long-term immune recovery is possible regardless baseline CD4+ T-cell count. However, it seems that the median number of CD4 T cells recovered at long term is around 200-300 cells/mm3 and the immune recovery slowed down after approximately 3 years of HAART and reached a plateau after 4-5 years of HAART. Moreover, patients who started therapy with a CD4+ T-cell count <200 cells/mm3 had a higher risk of having a last determination of CD4+ T-cell count low enough to allow the development of some opportunistic infections than patients with a higher level of baseline CD4+ T-cell count.
|
|
| |
| |
|
|
|