| |
Pancreatic toxic effects associated with co-administration of didanosine and tenofovir in HIV-infected adults
|
| |
| |
Esteban Martínez, Ana Milinkovic, Elisa de Lazzari, Giovanni Ravasi, José L Blanco, Maria Larrousse, Josep Mallolas, Felipe García, José M Miró, José M Gatell
Infectious Diseases Unit, Hospital Clínic, University of Barcelona, Calle Villarroel 170, 08036 Barcelona, Spain (E Martínez MD, A Milinkovic MD, E de Lazzari BSc, G Ravasi MD, J L Blanco MD, M Larrousse MD, J Mallolas MD, F García MD, J M Miró MD, Prof J M Gatell MD)
Lancet 2004; 364: 65-67
See Commentary Below by Graeme Moyle & Maria Boffito
"...All six patients with symptomatic pancreatitis were women, without renal impairment, weighing between 47 and 56 kg, and they had been given either 250 mg or 400 mg of didanosine... Since pancreatitis did not develop in men, in patients who weighed more than 60 kg, or those given tenofovir alone, these variables were excluded from the multivariate analysis... The incidence in people weighing 60 kg or less (n=179) was 2·9 (1·3-6·5), and in those greater than 60 kg (n=396) it was 0 (0-0·8)... In patients receiving didanosine plus tenofovir, the incidence of pancreatitis in those weighing 60 kg or less (n=62) was 7·3 (3·0-17·4), in women (n=37) it was 10·9 (4·5-26·2), and in women weighing 60 kg or less (n=31) it was 13·3 (5·6-32·1)..."
SUMMARY. The rise in didanosine concentrations in plasma when given with tenofovir raises concern for a high risk of toxic effects. Recommendations to reduce didanosine dose have been issued, but only for adults weighing more than 60 kg. We reviewed cases of pancreatitis in patients receiving didanosine plus tenofovir, didanosine alone, and tenofovir alone to assess the incidence of and risk factors for pancreatitis. Between Aug 1, 2001, and Nov 30, 2003, five of 185 (2·7%) patients receiving didanosine plus tenofovir, one of 182 (0·5%) on didanosine without tenofovir, and none of 208 on tenofovir without didanosine developed pancreatitis (p=0·016). Co-administration of both drugs versus each of them individually was an independent risk factor for pancreatitis (crude hazard ratio 10·666, 95% CI 1·246-91·294, p=0·031). These results suggest that the risk of pancreatitis is heightened when didanosine and tenofovir are given together.
Simple regimens with few toxic effects are needed to maintain long-term success of antiretroviral treatment. Co-administration of didanosine and tenofovir has gained popularity because both can be taken as single pills, once daily, without food restrictions. An early pharmacokinetic study of the co-administration of these drugs reported a 40% rise in didanosine plasma concentrations, which has raised concern over potential high risk of didanosine toxic effects. However, the clinical significance of this pharmacokinetic effect is not known because intracellular didanosine concentrations do not always correlate with plasma concentrations.
Data from two 24-week placebo-controlled tenofovir trials, which included 197 patients receiving didanosine, have been assessed. Comparisons of toxic effects between groups receiving didanosine plus tenofovir, and didanosine plus placebo, showed no difference in the frequency of pancreatitis. However, cases of severe pancreatitis, with didanosine and tenofovir given together, have been reported. Findings of a pharmacokinetic investigation of reduced daily dose of didanosine in combination with tenofovir (300 mg), showed that didanosine (250 or 200 mg) given with food resulted in didanosine exposures similar to that of didanosine (400 or 250 mg) given alone with no food. Many clinicians use these results in clinical practice, whereas others continue to use standard didanosine doses. Because we noticed several cases of pancreatitis in HIV-infected patients treated with didanosine plus tenofovir we decided to review cases of pancreatitis in patients receiving didanosine plus tenofovir, didanosine alone, and tenofovir alone to assess the incidence of and risk factors for pancreatitis, and the effect of these treatments on serum lipase and amylase concentrations.
Tenofovir was first used in our HIV clinic in August, 2001. By Nov 30, 2003, 575 patients had received either didanosine plus tenofovir, didanosine alone, or tenofovir alone for at least a week, as part of their antiretroviral regimens (table). Patients treated with didanosine plus tenofovir who weighed more than 60 kg (n=123) recieved didanosine 400 mg (n=43, 35%), 250 mg (n=78, 63%), or 200 mg (n=2, 2%), and those weighing 60 kg or less (n=62) received didanosine 400 mg (n=5, 8%), 250 mg (n=48, 77%), and 200 mg (n=9, 15%). For those treated with didanosine alone, the dose was adjusted for weight. Five (2·7%) patients given didanosine plus tenofovir, and one (0·5%) given didanosine alone developed pancreatitis after a median follow-up (IQR) of 22 (12-24) weeks. Symptoms were acute and included nausea, vomiting, and abdominal pain. These patients had neither history of previous pancreatitis, nor any apparent predisposing factor, with the exception of stable, mild hypertriglyceridaemia (<4 g/L) in one patient. All six patients with symptomatic pancreatitis were women, without renal impairment, weighing between 47 and 56 kg, and they had been given either 250 mg or 400 mg of didanosine. Other concurrent treatments in patients with pancreatitis included lopinavir plus ritonavir (n=1), lamivudine (2), and efavirenz (2) for those treated with didanosine plus tenofovir; and lamivudine plus nevirapine for the patient given didanosine alone. Pancreatobiliary imaging was normal and all patients recovered after stopping antiretroviral treatment. We noted no other didanosine-related clinical complications, such as polyneuropathy or lactic acidosis. Plasma lactate concentration was normal in two of the six patients in whom it was measured.
Baseline characteristics including sex, age, weight, CD4 cell count, plasma HIV-1 RNA, amylase, and lipase were similar in the three treatment groups (data not shown). There were substantial differences between patients with and without pancreatitis in nucleosides used, sex; and baseline weight, CD4 count (cells per µL), and amylase. Since pancreatitis did not develop in men, in patients who weighed more than 60 kg, or those given tenofovir alone, these variables were excluded from the multivariate analysis. Multivariate analysis of baseline values of CD4 cell count, HIV-1 RNA, amylase, and lipase showed that the only independent risk factor for pancreatitis was the number of CD4 cells per µL (crude hazard ratio 0·992 per unit increase, 95% CI 0·985-0·999, p=0·032). Multivariate analysis including patients taking didanosine alone and tenofovir alone, and grouped together, suggested that didanosine plus tenofovir was an independent factor for development of pancreatitis (crude hazard ratio 10·666, 95% CI 1·246-91·294, p=0·031).
Incidence (cases per 100 person-years, 95% CI) of pancreatitis in the overall population was 0·9 (0·4-2·0), whereas in those patients on didanosine plus tenofovir it was 2·3 (1·0-5·6), in those on didanosine without tenofovir it was 0·5 (0·1-3·3), and in those on tenofovir without didanosine it was 0 (0-1·6). The incidence in women was 4·0 (1·8-8·9), and in men it was 0 (0-0·7). The incidence in people weighing 60 kg or less (n=179) was 2·9 (1·3-6·5), and in those greater than 60 kg (n=396) it was 0 (0-0·8). By didanosine dose, the incidence in patients on didanosine 400 mg daily was 0·4 (0·1-2·9), in those on 250 mg daily it was 2·9 (1·2-6·9), and in those on 200 mg daily it was 0 (0-0·8). In patients receiving didanosine plus tenofovir, the incidence of pancreatitis in those weighing 60 kg or less (n=62) was 7·3 (3·0-17·4), in women (n=37) it was 10·9 (4·5-26·2), and in women weighing 60 kg or less (n=31) it was 13·3 (5·6-32·1).
Although amylase and lipase concentrations remained within normal ranges (<400 U/L and <200 U/L, respectively) in most patients, both enzymes were higher after patients were given didanosine plus tenofovir than with didanosine alone or tenofovir alone. In patients given didanosine plus tenofovir, lipase was paradoxically higher in those who had daily didanosine doses of either 250 mg or 200 mg than in those given 400 mg (figure). Increased concentrations of amylase or lipase, above the normal range, either at baseline or at 3 months were not predictive of clinical pancreatitis.
The present didanosine summary of product characteristics (February, 2003) suggests that appropriate doses for the combination of didanosine and tenofovir, with respect to effectiveness and safety, have not been established. The tenofovir summary of product characteristics was revised in August, 2003, to include a dose recommendation of 250 mg didanosine when co-administered with tenofovir in adults weighing more than 60 kg, although it notes that there are no available data to recommend didanosine dose adjustments for patients weighing less than 60 kg. Our data suggest that didanosine, even at a dose of 250 mg daily, when given with tenofovir is associated with increased risk of pancreatitis, especially in women weighing 60 kg or less.
COMMENTARY
Unexpected drug interactions and adverse events with antiretroviral drugs
Esteban Martinez and colleagues, in today's Lancet, report cohort data indicating that the combination of didanosine with tenofovir results in a significantly higher incidence of pancreatitis than regimens containing didanosine or tenofovir alone. Pancreatitis events, as well as hyperlipasaemia and hyperamylasaemia, were seen in patients taking 400 mg or 250 mg didanosine with tenofovir.
Drug toxicity, medication adherence and resistance emergence are the main obstacles to long-term therapy for HIV infection. Adverse effects of antiretroviral agents may be considered early (occurring within the first 3-6 months of therapy) or late (occurring in individuals who are established on and tolerating a drug for some time). Most early toxicities, such as nausea and diarrhoea, rash, and sleep disturbances, are predictable, transient, and of mild to moderate intensity. These adverse events are generally manageable with advice, palliative drugs, and only occasionally, such as with systemic hypersensitivity reactions, require treatment modification. Late side-effects, such as anaemia, peripheral neuropathy, pancreatitis, lipoatrophy, and lactic acidosis, whilst occurring in only a few individuals, are less amenable to management with palliative drugs and generally lead to therapy modifications or treatment interruption. Additionally, some of these late side-effects are potentially life-threatening or can lead to permanent disability or stigmatising morphological changes. It is therefore prudent to avoid drug combinations that are associated with an increased relative risk of late side-effects.
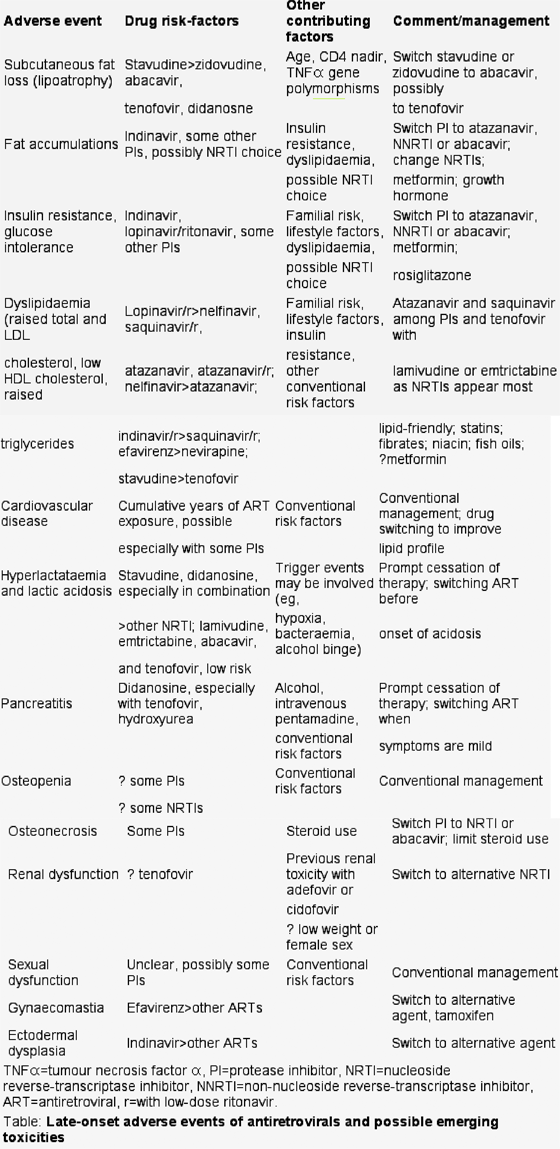
Many of these late side-effects have risen to prominence and their cumulative prevalence appreciated only in recent years as large numbers of people in the developed world have received chronic antiretroviral therapy and have been relatively free of morbidities related to HIV infection. In an era of therapy where HIV is controlled by medication it is adverse events secondary to antiretrovirals that provide the greatest burden of morbidity and possibly mortality for persons with HIV. The table shows some of the late toxicities of antiretroviral therapy that have risen to prominence in recent times and the agents with the possible greatest relative risk.
|
|
| |
| |
 |
|
| |
| |
The combination of stavudine with didanosine is associated with an increased relative risk of peripheral neuropathy, hyperlactataemia and lactic acidosis, and a more rapid loss of subcutaneous fat during therapy, compared with regimens that contain one of these agents combined with lamivudine or emtricitabine or regimens that do not include stavudine. The mechanism of several of these toxicities is thought to be related to inhibition of mitochondrial DNA-polymerase gamma. These problems have led to some national HIV-treatment guidelines recommending against the use of stavudine plus didanosine or indeed any stavudine-based regimen. Newer nucleoside reverse-transcriptase inhibitors, such as abacavir, lamivudine, tenofovir, and emtricitabine, have a much lower affinity to DNA polymerase gamma compared with zidovudine and dideoxy-nucleoside reverse-transcriptase inhibitors (stavudine, didanosine, zalcitabine). These agents have generally not been associated with clinical toxicities suggestive of mitochondrial toxicity.
The occurrence of pancreatitis with didanosine was noted during the development of this drug, although peripheral neuropathy was the dose-limiting toxicity. The original approved dose of didanosine for individuals who weighed over 60 kg was 750 mg a day. Subsequent studies showed that lower doses of this formulation of didanosine were similarly effective but better tolerated. In particular, pancreatitis occurred in 10% of patients on the high dose of didanosine monotherapy (750 mg a day) in ACTG116 a and 116b/117, but in only 1·1% of recipients of 400 mg a day as monotherapy in the ACTG175. The combination of didanosine with zidovudine in the ACTG 175 and DELTA studies resulted in pancreatitis rates of 0·2% and 1·1%, respectively. These rates were no different to those observed in the zidovudine-monotherapy arms. Diabetes mellitus has also been reported with didanosine, possibly as a consequence of pancreatitis.
The mechanisms by which didanosine triggers pancreatitis are not known but may be influenced by its metabolism through the purine pathway. Chronic exposure to didanosine in rodents does not have direct toxic effects on the pancreas. The role of free radicals in the pathogenesis of pancreatitis has been shown with a range of chemicals. Didanosine is metabolised to hypoxanthine, xanthine, uric acid, and allantoin. Xanthine oxidase is an important generator of free radicals, which suggests that the increased activity of this enzyme secondary to didanosine metabolism might be the source of pancreatitis- inducing free radicals, A contribution of mitochondrial toxicity cannot be ruled out, although in-vitro didanosine (or its active form ddATP) has no effect on the ATP content of pancreatic cells.
A significant interaction exists between didanosine and tenofovir. An overall increase in circulating levels of didanosine (44-60% regardless of didanosine's formulation and the patient's fed or fasted state) indicates the potential for an increased risk of didanosine-mediated adverse events. Use of a reduced dose of enteric-coated didanosine (250 mg in patients weighing over 60 kg) produces plasma concentrations of didanosine similar to those with the standard dose of 400 mg in the absence of tenofovir. However, the high inter-individual variability in plasma concentrations of didanosine might not exclude the achievement of toxic exposures to didanosine despite the administration of lower doses of the drug, and might risk subtherapeutic exposure to didanosine. Indeed, in one small comparative study, the relative risk of early virological failure was increased in treatment-naive patients with high baseline viral-loads and CD4 counts below 200/µL who started on didanosine with tenofovir, and efavirenz.17 Inhibition of purine nucleoside phosphorylase by tenofovir monophosphate or diphosphate is probably the mechanism.18 Although both agents are active as adenosine analogues, there is no evidence of intracellular interaction between didanosine and tenofovir in vitro.
Unexpected drug interactions between antiretroviral agents might lead to an increased risk of adverse events and of virological failure. Wherever feasible, novel antiretroviral combinations should be tested in drug-interaction studies and in adequately powered and carefully monitored clinical trials before becoming widely used in clinical practice.
Graeme Moyle, Marta Boffito
Chelsea and Westminster Hospital, London SW10 9NH, UK
|
|
| |
| |
|
|
|