| |
CCR5 Inhibitor Prevents HIV transmission in Monkeys
|
| |
| |
Microbicide Shuts the Door on HIV
Jon Cohen, Science, Oct 15, 2004
Microbicides have long had a stepchild status in the AIDS research community. Industry has had little interest in developing a topical gel or cream that can stop HIV at the vagina or rectum, and the products that have moved furthest in human studies are soaps and other substances that do not specifically target the virus. But over the past few years, nonprofits and governments have poured substantial money into microbicide research and development, bringing forward several cutting-edge concepts. On page 485 of this issue, an international team of researchers describes a monkey study that features one such strategy: a microbicide specifically designed to block HIV's ability to infect its favorite target cell. "They are applying true antiretroviral science to microbicides," says Mark Mitchnick, who heads R&D for the nonprofit International Partnership for Microbicides in Silver Spring, Maryland.
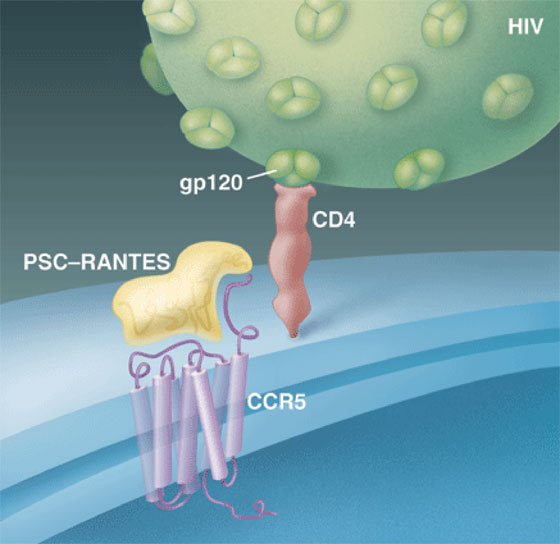
HIV typically establishes an infection by first attaching to CD4 receptors on white blood cells and then grabbing a second receptor known as CCR5, which normally responds to immune chemicals called chemokines. In the study, clinical immunologist Michael Lederman of Case Western University in Cleveland, Ohio, teamed up with Oliver Hartley of the University of Geneva in Switzerland, whose lab had created a CCR5 inhibitor, PSC-RANTES, by modifying one of the chemokines that uses the receptor. Working with a group led by Ronald Veazey of the Tulane National Primate Research Center in Covington, Louisiana, they applied different doses of the compound to the vaginas of 30 monkeys. Fifteen minutes later, they challenged the animals with an intravaginal dose of a chimeric monkey/human AIDS virus. In animals given relatively high doses of PSC-RANTES, 12 of 15 completely resisted infection. "This is the first paper that says if you target the susceptible cells, you can block infection by mucosal cells," says Robin Shattuck of St. George's Hospital Medical School in London.
Blocked dock. PSC-RANTES prevents infection of CD4 cells by blocking HIV's gp120 from binding to CCR5--
|
|
| |
| |
 |
|
| |
| |
Many mysteries remain about the mechanism of sexual transmission of HIV, and Lederman suggests that this study may help clear up a critical one. Although other studies have shown sexual transmission of the virus through routes that don't involve the CD4/CCR5 nexus, "this experiment suggests that blocking CCR5 is enough to prevent infection," says Lederman.
Yet he is quick to point out that the dose of PSC-RANTES required for protection in this study is "too high to be practical." Manufacturing the amount of PSC-RANTES needed to protect each monkey proved extremely expensive, so the Geneva team is now attempting to develop a cheaper version of the molecule. Lederman and others also note that several companies have developed potentially cheaper, small-molecule CCR5 inhibitors. Veazey, working with AIDS immunologist John Moore of Cornell University's Weill Medical College in New York City, last year found that one of these protected two of 11 monkeys in a viral challenge experiment. "We've done better since," says Moore.
Lederman and colleagues also raise the possibility that their study may have set the bar too high; the monkeys were given hormones to make them more susceptible to the virus. Smaller amounts of PSC-RANTES might therefore work in the real world. Some human studies have shown that the transmission of HIV from male to female may occur as infrequently as one out of every 2000 sexual encounters. But a group led by Christopher Pilcher of the University of North Carolina, Chapel Hill, published a study in the May issue of the Journal of Infectious Diseases reporting that males in the initial stage of an HIV infection can transmit as frequently as once out of every four encounters.
Shattuck says it should be assumed that a microbicide will have to protect against high-dose challenges. Still, he is heartened by the new study. "We've moved from an era of trying unsophisticated approaches to rational drugs that we understand," Shattuck says. "It's a new phase in microbicide approaches."
Prevention of Vaginal SHIV Transmission in Rhesus Macaques Through Inhibition of CCR5
Science, 15 October 2004
Michael M. Lederman,1*{dagger} Ronald S. Veazey,2* Robin Offord,3* Donald E. Mosier,4 Jason Dufour,2 Megan Mefford,2 Michael Piatak, Jr.,5 Jeffrey D. Lifson,5 Janelle R. Salkowitz,1 Benigno Rodriguez,1 Andrew Blauvelt,6{ddagger} Oliver Hartley3
Topical agents, such as microbicides, that can protect against human immunodeficiency virus (HIV) transmission are urgently needed. Using a chimeric simian/human immunodeficiency virus (SHIV SF162), which is tropic for the chemokine receptor CCR5, we report that topical application of high doses of PSC-RANTES, an amino terminus--modified analog of the chemokine RANTES, provided potent protection against vaginal challenge in rhesus macaques. These experimental findings have potentially important implications for understanding vaginal transmission of HIV and the design of strategies for its prevention.
1 Department of Medicine, Case Western Reserve University, University Hospitals, 2061 Cornell Road, Cleveland, OH 44106, USA.
2 Tulane National Primate Research Center, 18703 Three Rivers Road, Covington, LA 70433, USA.
3 Department of Structural Biology and Bioinformatics, Faculty of Medicine, University of Geneva, 1211 Geneva 4, Switzerland.
4 Scripps Research Institute, 10550 Torrey Pines Road, La Jolla, CA 92037, USA.
5 AIDS Vaccine Program, SAIC Frederick, Inc., National Cancer Institute, Frederick, MD 21702-1201, USA.
6 Dermatology Branch, National Cancer Institute, Bethesda, MD 20892--1908, USA.
Because the vast majority of HIV infections are acquired via transmission across mucosal surfaces, strategies to prevent mucosal transmission are urgently needed. Unfortunately, the mechanisms whereby HIV gains entry at mucosal sites, especially vaginal sites of infection, are incompletely understood. Thus, there is no uniform agreement regarding the critical host cellular and molecular targets during infection after vaginal exposure, and resolution of these issues is needed for the design of plausible microbicide strategies to prevent mucosally acquired HIV infection.
The chemokine receptor CCR5 serves as an essential cofactor for HIV entry and acquisition of infection. Thus, persons whose cells lack surface CCR5 expression because of mutation are almost completely protected from acquiring HIV infection. Furthermore, viruses that utilize CCR5 predominate in early stages of mucosal transmission, which suggests that mucosal transmission may selectively involve CCR5. Hence, inhibition of CCR5 has been proposed as a possible "microbicide" strategy for prevention of HIV infection.
However, HIV is able to use other host cell factors that are present at mucosal sites to achieve or to facilitate infection. These findings have led to some debate about the importance of CCR5 for infection across mucosae, as well as concern that targeting CCR5 alone may be inadequate to prevent transvaginal HIV transmission.
We previously described the synthesis of an amino terminus--modified form of the chemokine RANTES, the aminooxypentane oxime of [glyoxylyl1]RANTES, known as AOP-RANTES. This compound is significantly more potent at inhibiting HIV-1 replication than the parent chemokine. Subsequently, a series of amino-terminally modified RANTES analogs have been developed and tested in an effort to improve potency and durability of HIV inhibitory activity.
AOP-RANTES blocked in vitro propagation of multiple CCR5-using HIV isolates representing clades A to F with inhibitory concentrations in the nanomolar range. Pretreatment of hu-PBL-SCID chimeras (mice with severe combined immunodeficiency disease reconstituted with human peripheral blood lymphocytes) with another more potent RANTES analog, N{alpha}-(n-nonanoyl)-des-Ser1-RANTES (known as NNY-RANTES) protected animals from parenteral HIV challenge, although in some, escape with both R5- and X4-using viruses was demonstrated.
A third analog, N{alpha}-(n-nonanoyl)-des-Ser1-[L-thioproline2, L-{alpha}-cyclohexyl-glycine3]RANTES (PSC-RANTES) represents a new RANTES analog chemically identical to native RANTES except for the substitution of a nonanoyl group, thioproline, and cyclohexylglycine for the first three N-terminal amino acids of the native protein. PSC-RANTES has more potent in vitro antiviral activity than earlier analogs, with inhibitory concentrations for some HIV-1 isolates in the picomolar range. The induction of receptor internalization and down-modulation on binding by chemokines is believed to play a major role in their anti-HIV action, although some contribution from competitive binding cannot be excluded. Our RANTES analogs show a particularly enhanced capacity to induce such internalization and down-modulation, and this may be the basis of their potent anti-HIV activity.
We first confirmed that PSC-RANTES inhibited propagation of the SHIV SF162 R5-tropic virus in rhesus peripheral blood mononuclear cells (PBMCs) and completely blocked SHIV SF162 replication, with median inhibitory concentration (IC50) values in the subnanomolar range. Furthermore, PSC-RANTES caused down-modulation of macaque CCR5. After only 15 min of exposure, the decrease in CCR5 levels on both CD4-- and CD4+ peripheral blood cells was already maximal [>90%].
To examine PSC-RANTES' ability to prevent acquisition of SHIV infection at a mucosal site, 30 progesterone-treated adult female rhesus macaques were pretreated with 4 ml PSC-RANTES at the indicated concentrations or with phosphate-buffered saline (PBS). The animals were subsequently challenged with a high multiplicity [300 TCID50 (median tissue culture infectious dose)] of SHIV SF162 and monitored for up to 24 weeks for the development of plasma viremia (29). All five animals treated with the highest dose (1 mM) of PSC-RANTES were protected from SHIV infection, with no detectable viremia for the entire duration of follow-up. Lower doses also proved protective, with four out of five animals treated with 330 µM and three out of five treated with 100 µM PSC-RANTES also showing protection from infection. One of five animals treated with 33 µM PSC-RANTES and two of five animals treated with 10 µM or less were protected. Overall, 12 out of 15 animals (80%) pretreated with >=100 µM were protected from infection, whereas only 4 out of 15 animals (27%) pretreated with <100 µM or placebo showed protection [P = 0.009, Fisher's exact test; risk ratio (RR) = 0.27, 95% confidence interval (CI), 0.09 to 0.78 (27)]. There was a significant dose-effect relationship when considering the whole range of dosing levels (P = 0.0048, Cochran-Armitage exact trend test). Based on exact logistic regression modeling, we estimated that in this system a 10-fold increase in PSC-RANTES dose was associated with an odds ratio (OR) of infection of 0.39 [95% CI, 0.17 to 0.82], (P = 0.035).
Plasma samples obtained at week 11 from all protected animals and four infected animals were tested for the presence of antibodies to simian immunodeficiency virus (SIV) proteins by Western blot. All four infected animal plasmas had strong bands corresponding to p17, p27, p55, and p66, whereas no positive bands were found in any of the protected animals. Plasma levels of PSC-RANTES were measured in samples obtained at intervals (1 hour, 4 hours, and 24 hours) after intravaginal administration of the maximum concentration used (1 mM). PSC-RANTES was undetectable (lower than the 300 pM limit of assay sensitivity) in all samples tested.
We have shown that PSC-RANTES, targeting CCR5 alone, protected rhesus macaques from intravaginal exposure to a chimeric SHIV containing an R5-tropic envelope of HIV-1. Thus, pursuing a strategy that targets this receptor seems reasonable for development. However, the concentrations used in the highest dose group (1 mM), exceed by orders of magnitude the subnanomolar IC50 of our agent against SHIV SF162. Recently, in the same animal model, the neutralizing antibody IgG1-b12 gave partial protection against vaginal transmission of SHIV, also at concentrations vastly in excess of those needed in vitro. A small-molecule CCR5 inhibitor, highly potent in vitro, gave only minimal protection in the animal system used here, even as a virtually saturated solution. Cyanovirin partially inhibited vaginal transmission of a SHIV isolate that also targets CXCR4 but only at concentrations ~10,000 times those required for full inhibition in vitro. Possible explanations for these dose disparities include incomplete distribution, failure to penetrate to hypothetical submucosal target sites, nonspecific adsorption to mucosal surfaces, or degradation or inhibition by vaginal factors. But conceivably, the explanation might simply be that the progesterone treatment and the dose of SHIV that we and others use to ensure near-universal infection of control macaques [28 of 31 in this system] constitute an extraordinary challenge. In the natural human setting, risk for acquisition of HIV infection after sexual exposure, although probably not uniform, is on average markedly lower than that shown by unprotected controls in this animal system. Given the discrepancy between in vitro and in vivo potency, we offer our findings as a proof of principle and as a direction for attempts to render the approach economically acceptable.
PSC-RANTES protected macaques from intravaginal challenge without detectable toxicity or histological changes. Consequently, further development of this and related compounds, either alone or in combination with other agents, and improvement of their formulation are reasonable subjects for further study as an approach to the prevention of sexual transmission of HIV.
|
|
| |
| |
|
|
|