| |
Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome
|
| |
| |
AIDS: Volume 18(17) 19 November 2004
Puoti, Massimo; Bruno, Raffaelea; Soriano, Vincentb; Donato, Francescoc; Gaeta, Giovanni Battistad; Quinzan, Gian Paoloe; Precone, Davidef; Gelatti, Umbertoc; Asensi, Victorf; Vaccher, Emanuelag; for the HIV HCC Cooperative Italian-Spanish Group
From the Clinica di Malattie Infettive, Università degli Studi di Brescia-AO Spedali Civili, Brescia, aClinica di Malattie Infettive e Tropicali; Università di Pavia, Pavia, Italy, bDepartment of Infectious Diseases, Hospital Carlos III, Madrid, Spain, cIstituto d'Igiene, Università degli Studi di Brescia; Brescia, dDipartimento Malattie Infettive, SUN, Napoli, eDivisione di Malattie Infettive Ospedali Riuniti, Bergamo, Italy, fInfectious Diseases Unit, Hospital General, Oviedo, Spain, and the CRO Aviano, Italy. *The HIV-HCC Cooperative Italian-Spanish group was a cooperation between four multicentre study cohorts (see Appendix).
"...Among the 41 HIV-infected patients with HCC, 35 (85%) were former IDU, three were men who had had sex with other men, two admitted multiple heterosexual contacts and one had been infected by blood transfusions in the early 1980s. At the time of first diagnosis of HCC, the median time from first parenteral risk exposure was 22 years. Nine (24%) HIV-infected patients had previously being diagnosed of an AIDS-defining illness. The median CD4 T-cell count at diagnosis of HCC was 267, Interestingly, 25 (61%) had CD4 cell counts > 200 and eight (20%) had CD4 cell counts > 350. Thirty-one patients (77%) were on highly active antiretroviral therapy. Plasma HIV-RNA levels at the time of HCC diagnosis were available for 38 out of 41 patients; undetectable viraemia was recorded in 16 (42%) of them. In the rest, low-level viraemia (< 10,000 HIV-RNA copies/ml) were recorded in 18 (47%) and only four (10%) showed higher plasma HIV-RNA titres. All patients taking HAART showed plasma HIV-RNA levels < 10,000 copies/ml..."
Abstract
Objective: Hepatocellular carcinoma (HCC) is an increasing cause of mortality in HIV-seropositive individuals. The aim of the study was to compare the main features of HCC in HIV-seropositive individuals with those in to HIV-negative patients.
Patients: and methods: All HIV-infected subjects with a diagnosis of HCC included in three cancer registry databases were enrolled in the study as cases. HCC cases that occurred in the province of Brescia, North Italy, in the period 1995-1998 and all cases reported at the Italian Liver Cancer Project were enrolled as controls. All data were collected using a standardized case report form. The main clinical and epidemiological characteristics of patients with HCC and their survival were compared between HIV-positive and uninfected subjects.
Results: Forty-one HIV-infected subjects with HCC were identified. Multivariate analysis adjusted for age and sex identified an association between HIV infection and HCV infection [odds ratio (OR), 11; P = 0.005], and infiltrating tumours and/or extranodal metastasis at presentation (OR = 11.8; P < 0.001). HIV infection was independently associated with shorter survival (hazard ratio, 1.63; P = 0.015).
Conclusions: HCC in HIV-infected patients is mainly associated with underlying chronic hepatitis C and has a more aggressive clinical course. Thus, preventative strategies (including the treatment of hepatitis C) should be implemented in the management of HIV/HCV-coinfected patients.
Author Discussion
This is the largest series of HCC reported so far in HIV-infected individuals. Previous reports had recorded anecdotal cases or small series. In agreement with many of them, we found that HCC in most instances presented in patients with well-controlled HIV disease. Only one-third of individuals had end-stage liver disease (Child-Pugh class C cirrhosis) and all but one of the 31 who died during follow-up had liver disease as the main cause of death. Therefore, in most cases, progression of HCC was the main determinant of prognosis in these patients. In fact most of HIV-infected patients had well-controlled HIV replication and stable HIV disease so the comorbidity and mortality due to HIV infection per se was minimal in this group of patients. On the other hand survival induced by successful antiretroviral therapy has probably allowed the risk of HCC to be expressed.
To investigate possible differences between HCC in HIV-infected patients and HIV-uninfected individuals, we examined two of the largest databases of HCC in HIV-negative patients available in Southern Europe, where all our HIV-positive patients with HCC were recognized. To make the comparisons more reliable, all data were collected using the same case record form as in the prospective data registry of the Brescia HCC Study Group. Furthermore, a second independent control group including all HCC cases recruited by the CLIP Study Group was used. Age, sex and rate of hepatitis C were the most significant differences found when comparing HIV-positive and uninfected patients with HCC. The former were significantly younger, almost all were male and had underlying chronic hepatitis C more frequently than the latter. These three characteristics are somewhat related, as it is well known that IDU drug users represents a large portion of HIV-positive individuals in Italy and Spain, most of whom are male and HCV-coinfected.
The median age of HIV-infected patients at first diagnosis of HCC was 42 years, significantly younger than that seen in population-based cancer registries and other series of HCC from non-developing countries, especially in the Mediterranean basin where HCV and HBV infections as well as heavy alcohol intake are responsible for almost all HCC cases. The early age at HCC diagnosis in HIV-infected subjects could be attributed to their young age at the time of exposure to HCV infection, on average in their early 20s, as a consequence of needle sharing using with other IDU. In addition, a more rapid course of HCV-related liver disease in HIV-coinfected subjects could also have played a role. Furthermore, an enhanced hepatocarcinogenesis related to extrahepatic growth signals from the HIV tat gene-expressing cells has been reported in the transgenic mice.
The earlier occurrence of HCC in HIV/HCV-coinfected patients is supported by the finding of a median time since first estimated parenteral exposure to HCV of 22 years, which is at least 10 years shorter than that reported in series of HCC in HIV-negative patients with post-transfusion hepatitis C. This shorter incubation period for HCC in HIV-infected patients should alert us of a possible increase in the occurrence of HCC in Southern Europe within the next years, as HCV/HIV coinfection is quite prevalent. In accordance with this presumption, a fivefold increase in HCC as a cause of liver-related death among HIV-infected patients has been noticed in France during the period 1995-2001.
The higher prevalence of HCV infection among HIV-infected patients as compared with HIV-uninfected HCC patients should be emphasised. It suggests that we should assume more aggressive attitudes to the treatment of hepatitis C in HCV/HIV-coinfected patients. Moreover, up to one-third of our HIV-positive patients with HCC had multiple causes of liver disease. Both hepatitis B and high alcohol intake enhance the risk of HCC in patients with chronic hepatitis C. Therefore, prevention and treatment of HBV infection and efforts to avoid alcohol consumption in the HCV/HIV-coinfected should be considered a priority for preventing HCC in this population.
Other relevant data emerging from our study was the recognition of a more aggressive course of HCC in HIV-infected patients. We found an independent association between HIV infection and a more advanced stage of HCC at clinical presentation, with a higher rate of infiltrating neoplasms and extrahepatic-extranodal metastases. Furthermore, HIV infection was independently associated with a reduced survival rate with respect to HCC in HIV-negative patients.
Advanced HCC at clinical presentation and shorter survival could be related to a delayed diagnosis of HCC in HIV-infected patients. However, we did not find a different rate of advanced neoplasia or a different survival in the subset of HIV-positive patients who showed negative liver ultrasound examination 6 months before HCC diagnosis. Therefore, the more aggressive clinical course of HCC in HIV-positive patients could be due to specific HIV-related features, including the presence of growth signals enhancing HCC cell proliferation and/or a weaker anti-tumour immune response, as our recognition of an association between HCC invasiveness and lower CD4 cell counts seemed to suggest.
One limitation of our study is the retrospective design. To our knowledge, there are no data reporting the incidence of HCC in longitudinal prospective series of HIV-infected patients. Until those results are forthcoming, we feel our results are quite reliable as the patients were recruited consecutively and the revision of HCC diagnosis was made using stringent standardized criteria, which should have minimized biases.
In conclusion, we describe a relatively large series of HCC in HIV-infected patients. They seem to be mostly due to HCV infection. The younger age of these patients as compared with HIV-negative patients with HCC and the shorter interval between the estimated date of first HCV exposure and first diagnosis of HCC should be highlighted; hepatocarcinogenesis could be a more rapid process in HIV/HCV-coinfected patients and, therefore, an increasing number of cases should be expected within the the next few years in this population. On the other hand, the clinical course of HCC seems to be more aggressive in HIV-positive patients, making the possibility of more effective treatment less likely for them. Therefore, prevention strategies, including more frequent abdominal ultrasound screening and AFP testing are advisable, in order to allow diagnosis at a less advanced stage which may permit the use of surgical or loco-regional treatment. Furthermore, prevention and treatment of hepatitis C in coinfected patients, and efforts to reduce alcohol abuse and prevent hepatitis B infection (i.e., by vaccination) should be encouraged.
RESULTS
Main demographics of HIV-positive patients with HCC
In the group of HIV-infected subjects, 41 cases of HCC were recorded. They were diagnosed in 1989 (n = 1), 1997 (n = 2), 1998 (n = 2), 1999 (n = 2), 2000 (n = 6), 2001 (n = 18) and 2002 (n = 10). Thirty-three cases were recorded in Italy and eight in Spain.
HIV-infected patients with HCC were significantly younger [median age, 42 years; interquartile range (IQR), 40-46 years; range, 26-61 years] than HIV-negative counterparts in the Brescia HCC Study Group (median age, 65 years; IQR, 60-70 years; range, 27-76 years) (P < 0.0001). The former group included a significantly higher proportion of males than the latter (98% versus 82.3%; P = 0.02). Similarly, HIV-infected patients were younger and more often male than the 701 cases of HCC from the CLIP registry (median age, 64 years; IQR, 59-70 years; range, 27-89 years; male, 75.3%; P = 0.002).
Among the 41 HIV-infected patients with HCC, 35 (85%) were former IDU, three were men who had had sex with other men, two admitted multiple heterosexual contacts and one had been infected by blood transfusions in the early 1980s. At the time of first diagnosis of HCC, the median time from first parenteral risk exposure was 22 years (IQR, 18-25 years; range, 11-31 years). Nine (24%) HIV-infected patients had previously being diagnosed of an AIDS-defining illness. The median CD4 T-cell count at diagnosis of HCC was 267 × 106cells/l (IQR, 180-341 × 106cells/l; range, 37 × 106-681 × 106cells/l). Interestingly, 25 (61%) had CD4 cell counts > 200 × 106cells/l and eight (20%) had CD4 cell counts > 350 × 106cells/l. Thirty-one patients (77%) were on highly active antiretroviral therapy. Plasma HIV-RNA levels at the time of HCC diagnosis were available for 38 out of 41 patients; undetectable viraemia was recorded in 16 (42%) of them. In the rest, low-level viraemia (< 10 000 HIV-RNA copies/ml) were recorded in 18 (47%) and only four (10%) showed higher plasma HIV-RNA titres. All patients taking HAART showed plasma HIV-RNA levels < 10 000 copies/ml.
Main features of HCC in HIV-infected patients
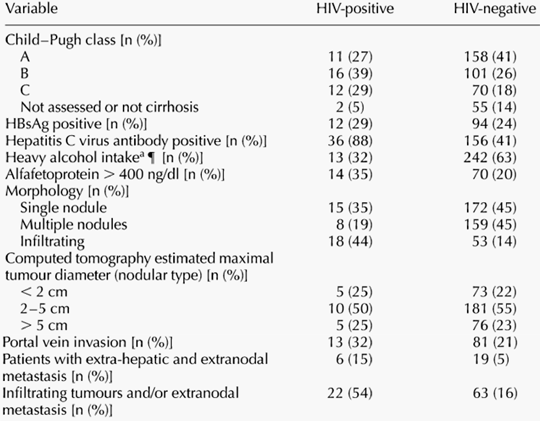
The main characteristics of the liver disease in the 41 HIV-infected patients with HCC and in the 384 HIV-uninfected controls with HCC from the Brescia HCC Study Group are recorded in Table 1. In patients with HIV infection, liver disease stage was significantly more advanced, and liver tumours had a significantly higher prevalence of multifocal and infiltrating lesions. Moreover, the proportion of HIV-positive patients with extranodal and extrahepatic metastases was much higher with respect to HIV-negatives.
Among the risk factors for liver disease, there was a higher rate of underlying HCV infection but a lower rate of heavy alcohol intake in HIV-infected patients as compared with HIV-uninfected patients with HCC. No difference was found in the rate of chronic HBV infection, AFP levels or the maximal diameter of the largest tumour nodule (Table 1).
Only three variables were significantly more frequent in HIV-positive versus HIV-uninfected patients with HCC, after adjusting for each other and for age and sex, using logistic regression analysis: a higher rate of chronic hepatitis C, a lower prevalence of heavy alcohol intake and a higher frequency of infiltrating and/or metastatic neoplasms at first HCC diagnosis.
Table 2 compares HIV-infected patients with HCC and the cases from the CLIP registry database. By univariate analysis, liver disease stage was more advanced, chronic hepatitis C more frequent and extrahepatic-extranodal metastases more common in HIV-positive than in HIV-uninfected patients, similar to what was seen in the analysis of the Brescia HCC registry. Furthermore, herein portal vein thrombosis was more frequent among HIV-infected patients with HCC. A higher rate of HCV infection and a higher proportion of cases with extrahepatic-extranodal metastases were the two variables independently associated with HCC in the HIV setting, using logistic regression analysis.
Patients with HIV infection were further divided into two groups, according to the presence of infiltrating liver tumours and/or extrahepatic-extranodal metastases. Twenty-two had 'invasive' HCC and 19 had less advanced cancer. A comparison between the two groups showed a lower absolute number of CD4 T cells among those with invasive tumours. Their median CD4 cell count was 210 × 106/l (IQR, 167-270 × 106/l) versus 350 × 106/l (IQR, 184-447 × 106/l) (P = 0.03) in patients with non-invasive tumours. No association was found between the presence of invasive tumours at presentation and the use of antiretroviral therapy nor with specific drugs such as protease inhibitors.
In order to assess the impact of delayed diagnosis on the clinical presentation of HCC among HIV-infected patients, we identified 22 out of the 41 patients who had Child-Pugh classes A or B suitable for HCC screening and who were periodically assessed with abdominal ultrasonography (US) and AFP measurement. Eight out of the 22 had undergone the last US no more than 6 months before first HCC diagnosis. Five out of eight periodically screened patients for HCC versus six out of 14 who were not under regular HCC screening showed a liver tumour with a diameter greater than 5 cm and/or with extrahepatic-extranodal metastases.
Treatment and follow-up of HCC
Twenty-five out of 41 (60%) HIV-infected patients received no treatment or only supportive therapy for HCC. This proportion was significantly higher than in HIV-negative patients with HCC enrolled in the Brescia HCC Study Group (38%) (P = 0.02) or in the CLIP registry (43%) (P = 0.053). Two out of 41 HIV-positive patients with HCC underwent hepatic resection and 14 (35%) non-surgical loco-regional treatment (percutaneous ethanol injection, radiofrequency ablation or transarterial chemoembolization). The median interval between completion of therapy and diagnosis of HCC was 3 months.
Two of the HIV-infected subjects were lost to follow up soon after the initial diagnosis and eight (19%) were alive at the end of follow-up. The median duration of follow-up was 182 days (IQR, 43-303 days; range, 10-1431 days). Proportional survival estimates for HIV-infected patients with HCC was 28% at 1 year (95% CI, 15-43%) and 11% at 2 years (95% CI, 2-26%). They were significantly lower than in HIV-uninfected patients with HCC from the Brescia HCC Study Group, which survival of 57% (95% CI, 52-62%) and 41% (95% CI, 36-46%) at 1 and 2 years, respectively.
Kaplan-Meier survival curves in HCC patients with and without HIV infection are shown in Fig. 1a. The predictors of survival in the multivariate analysis are shown in Table 3. HIV infection together with Child-Pugh stage, portal vein invasion, tumour diameter, serum AFP levels and any cancer therapeutic intervention were all significantly associated with longer survival. In contrast, age, HCV infection, history of alcohol abuse, sex and the presence of extranodal metastases were not associated with patients' survival.
Proportional survival was also significantly longer in HIV-negative patients from the CLIP database: 60% at 1 year (95% CI, 57-64%) and 40% at 2 years (95% CI, 37-44%). Kaplan-Meier survival curves for the 41 HIV-infected patients with HCC and the 701 from the CLIP database are shown in Fig. 1b. Herein, the predictors of longer survival in the multivariate analysis are recorded in Table 4.
Treatment for HCC was an independent predictor of survival in both analysis. The proportional survival of the 16 HIV infected patients who received treatment was 51% at 1 year (95% CI, 25-71%) and 41% (95% CI, 16-75%) at 2 years significantly longer than that observed in untreated HIV infected patients (11% at 1 year; 95% CI, 2-29%; no untreated patient was alive 2 years after diagnosis; P = 0.01, log-rank test). In untreated anti-HIV seronegative patients survival was 19% at 1 year (95% CI, 13-26%) and 9% at 2 years (95% CI, 5-14%); in treated anti-HIV seronegative patients it was 79% at 1 year (95% CI, 74-94%) and 60% after 2 years (95% CI, 53-66%). Among treated patients proportional survival was significantly longer in HIV uninfected patients (P = 0.04, log-rank test).
Table 1. Liver disease features at first diagnosis of hepatocellular carcinoma. Comparison between 41 HIV-infected and 384 uninfected patients with HCC living in the Brescia area (Brescia HCC study). Odds ratios (OR) for HIV infection status by univariate and multivariate analysis, using logistic regression analysis, adjusting the estimates for age and sex and each variable investigated.aHeavy alcohol intake is classed as a daily intake of > 60 g of ethanol per day for at least 10 years. CI, Confidence Interval; HBsAg, hepatitis B virus surface antigen.
|
|
| |
| |
 |
|
| |
| |
Introduction
Viral hepatitis and alcohol abuse are the main risk factors for hepatocellular carcinoma (HCC) in developed countries. Exposure to these risk factors is common among HIV-infected patients. Hepatitis C and B viruses (HCV and HBV) share the same transmission routes with HIV, and alcohol abuse is associated with HIV risk behaviours. In the EuroSIDA cohort, which comprises 3048 HIV-positive patients, the prevalence of HIV/HCV coinfection was 33%, but when only injecting drug users (IDU) were considered this proportion rose to 75%. In the USA around 200 000 people are HIV/HCV coinfected and, similarly, the highest rates of coinfection are seen among IDU. On the other hand, more than 80% of HIV-positive patients show markers of past or current HBV infection and up to 10% of them are chronic carriers of hepatitis B virus surface antigen (HbsAg).
There is evidence in vitro and in animal models to support a pivotal effect of HIV on hepatitis virus or alcohol-related hepatocarcinogenesis mediated by Tat protein. Accordingly, an increased risk for HCC has been independently associated with HIV infection in a study among patients with parenteral risk exposure. Moreover, a large French survey examining the causes of death in HIV-seropositive individuals identified an increase in HCV-related HCC as a cause of liver-related death. It represented 4.7% in 1995 but rose to 11% in 1997 and 25% in 2001.
The main characteristics of HCC in HIV-positive subjects and their survival have been poorly described so far. The case series of HCC in HIV-positive individuals published to date have not cumulatively exceeded 30 subjects. However, some reports have emphasized a more aggressive course with respect to HCC seen in HIV-negative individuals. Our study aimed to investigate the main clinical and epidemiological characteristics of HCC in HIV-infected patients as well as its prognosis.
Patients and methods
The Italian Cooperative Group on AIDS and Tumours (GICAT) has been collecting data on the epidemiological and clinical features of tumours occurring in HIV-infected patients since 1986. A total of 41 consecutive Caucasian subjects with a diagnosis of HCC were identified from the GICAT case series, and from the tumour case series of two Spanish referral HIV centres between 1986 and 2002. HCC cases from the GICAT registry were diagnosed in 10 centres in Italy.
The diagnosis of HCC was made according to the invasive and non-invasive criteria formulated by the European Association for the Study of the Liver. Briefly, cyto-histological diagnosis or a focal lesion of more than 2 cm in diameter in the setting of liver cirrhosis, showing evidence of arterial hypervascularization with coincident findings in at least two imaging techniques in the presence of alfaphetoprotein (AFP) levels < 400 ng/ml or using a single imaging technique in the presence of AFP levels >= 400 ng/ml.
A questionnaire was sent to the GICAT participating centres and to the two Spanish institutions involved in the study to collect retrospectively the following information concerning HIV disease and HCC diagnosis: HIV exposure category, HIV staging according to the 1993 Centers for Disease Control and Prevention classification criteria, CD4 and CD8 T-cell counts, plasma HIV-RNA levels, antiretroviral treatment history, and time from first parenteral risk exposure. In addition, the questionnaire included the following information on demographics and liver disease: age, sex, Child-Pugh score for cirrhosis, HBsAg, anti-hepatitis D virus (HDV), anti-HCV and serum HCV RNA, alcohol consumption, AFP levels, tumour morphology, presence of extranodal and extrahepatic metastases, and evidence of portal vein invasion. In addition, data on history of screening for HCC, any therapeutic intervention and follow-up after clinical presentation were collected retrospectively, and prospectively for those alive on January 2002.
The same information for liver disease was collected prospectively in 384 HIV-negative patients with HCC included in the registry from the Brescia Hepatocellular Carcinoma Study Group. All patients consecutively admitted to the two main hospitals in the province of Brescia, North Italy, with a first diagnosis of HCC between January 1995 and December 1998, and born in Italy, with complete clinical and pathological information were selected for this purpose. The main risk factors for HCC were assessed by testing for viral hepatitis markers and by interview, addressing specifically past exposure to alcohol.
In addition, for comparison purposes, we analysed another series of 701 patients with HCC recruited in the largest Italian study, the Italian Liver Cancer Program (CLIP), which includes first diagnosis of the disease in the period 1993-1998. The CLIP was settled in 1993, with the aim of performing prospective therapeutic trials and studies on diagnostic and prognostic assessment of HCC, mainly in Southern Italy. Data on alcohol consumption had not been systematically collected in that database.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and applicable local legislation. Local ethical committees of the participating centres approved the project and written informed consent was obtained from all subjects for the use of their clinical data for research purposes.
All of the clinical and pathological data and information on treatment for HCC were extracted from hospital clinical records. Survival was determined from the date of first diagnosis to the end of the follow-up period, which was 30 November 2003, and survival rates were estimated at 1 and 2 years.
|
|
| |
| |
|
|
|