| |
Reyataz Interaction with Prilosec & PPIs
|
| |
| |
Bristol-Myers Squibb distributed this Dear Doctor letter to doctors and clinicians.
"Re: REYATAZ® (atazanavir sulfate) With or Without Norvir (ritonavir) and Proton Pump Inhibitors Should Not Be Coadministered: Important New Pharmacokinetic Data"
December 2004
Dear Health Care Provider,
Bristol-Myers Squibb Company would like to make clinicians caring for HIV-infected patients aware of important new pharmacokinetic data concerning the coadministration of REYATAZ and Norvir (ritonavir, Abbott Laboratories, Inc.) with Prilosec® (omeprazole, AstraZeneca), a proton-pump inhibitor (PPI).
The following observations were made from a randomized, open-label, multiple-dose drug interaction study:
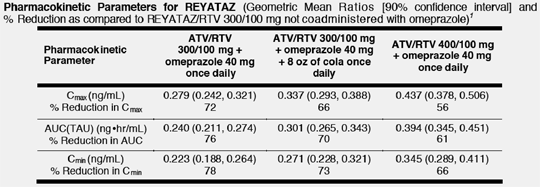
A 76% reduction in atazanavir area under the concentration-time curve (AUC) and a 78% reduction in atazanavir trough plasma concentration (Cmin) was observed when REYATAZ/ritonavir (RTV) 300/100 mg was coadministered with omeprazole 40 mg.
Based on the study results:
· DO NOT COADMINISTER REYATAZ OR REYATAZ/RTV with omeprazole due to the reduction in atazanavir exposure levels. This recommendation is consistent with the current REYATAZ U.S. Package Insert.
· It is not known whether the over-the-counter dose of omeprazole (20 mg once daily) would produce similar results; therefore, coadministration is not recommended.
· Increasing the REYATAZ/RTV dose to 400/100 mg in combination with omeprazole DID NOT result in REYATAZ exposures comparable to those observed with a regimen of REYATAZ 300/100 mg without omeprazole.
· Simultaneous administration of 8 ounces of cola given in an effort to decrease gastric pH did not appear to affect this reduction.
STUDY INFORMATION:
The table below shows data from a randomized, open-label, multiple-dose drug interaction study in healthy, HIV-uninfected subjects assessing comparability of the steady-state pharmacokinetics of REYATAZ/RTV 300/100 mg once daily (n=15), REYATAZ/RTV 300/100 mg with 8 ounces of cola once daily (n=15), and REYATAZ/RTV 400/100 mg once daily (n=14), each administered with omeprazole 40 mg once daily for 10 days. Subjects in each treatment arm received REYATAZ/RTV 300/100 mg for 10 days prior to randomization to one of the three treatment sequences and the addition of omeprazole on Day 10. In all cases, REYATAZ/RTV was administered with a light meal, and omeprazole was administered on an empty stomach 2 hours prior to REYATAZ/RTV.
Pharmacokinetic Parameters for REYATAZ (Geometric Mean Ratios [90% confidence interval] and % Reduction as compared to REYATAZ/RTV 300/100 mg not coadministered with omeprazole)1
|
|
| |
| |
 |
|
| |
| |
ATV = atazanavir, RTV = ritonavir, Cmax = peak plasma concentration, AUC(TAU) = area under the plasma concentration-time curve in one dosing interval, Cmin = trough plasma concentration.
Investigations regarding the potential drug interaction between REYATAZ (atazanavir sulfate) and H2-Receptor antagonists when coadministered are ongoing. Until data are available, clinicians should note the following statements from the REYATAZ Package Insert: "Reduced plasma concentrations of atazanavir are expected if H2-receptor antagonists are administered with REYATAZ (atazanavir sulfate). This may result in loss of therapeutic effect and development of resistance. To lessen the effect of H2 -receptor antagonists on atazanavir exposure, it is recommended that an H2-receptor antagonist and REYATAZ be administered as far apart as possible, preferably 12 hours apart."
Please refer to the accompanying REYATAZ Indication and Important Safety Information, and the enclosed Full Prescribing Information.
BMS is committed to providing you with current product information for the management of your patients with HIV infection. If you have any questions about this new information or require additional medical information, please contact the Virology Medical Services Department at Bristol-Myers Squibb Company at 1-800-426-7644 (select Option 3).
Sincerely,
Sally L. Hodder, MD
Vice President, Virology Medical Affairs
Bristol-Myers Squibb Company
REYATAZ is a registered trademark of Bristol-Myers Squibb Company. All other trademarks are the property of their respective owners and are not trademarks of Bristol-Myers Squibb Company.
Enclosures:
REYATAZ® (atazanavir sulfate) Package Insert
Important Information about REYATAZ
REFERENCE
1. Data on file, Bristol-Myers Squibb Company, Princeton, New Jersey.
Important Information about REYATAZ® (atazanavir sulfate) Capsules
INDICATION: Reyataz (atazanavir sulfate) is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection This indication is based on analyses of plasma HIV-1 RNA levels and CD4+ cell counts from controlled studies of 48 weeks duration in antiretroviral-naïve patients and antiretroviral-treatmentexperienced patients. The following points should be considered when initiating therapy with Reyataz :
· In antiretroviral-experienced patients with prior virologic failure, coadministration of Reyataz/ritonavir is recommended.
· In Study AI424-045 Reyataz/ritonavir and lopinavir/ritonavir were similar for the primary efficacy outcome measure of time-averaged difference in change from baseline in HIV RNA level. This study was not large enough to reach a definitive that REYATAZ/ritonavir and lopinavir/ritonavir are equivalent on the secondary outcome measure of proportions below the HIV RNA lower limit of detection.
· The number of baseline primary protease inhibitor mutations affects the virologic response to Reyataz/ritonavir.
· There are no data regarding the use of Reyataz/ritonavir in therapy-naïve patients.
IMPORTANT SAFETY INFORMATION:
· Coadministration with midazolam, triazolam, dihydroergotamine, ergotamine, ergonovine, methylergonovine, cisapride, or pimozide is contraindicated. Coadministration of REYATAZ with rifampin, irinotecan, lovastatin, simvastatin, proton-pump inhibitors, or St. John's wort (Hypericum perforatum)-containing products is not recommended. Voriconazole should not be administered to patients receiving REYATAZ/ritonavir. Caution should be used when prescribing sildenafil, vardenafil, or tadalafil with REYATAZ. This list of medications is not complete.
· PR interval prolongation has been observed in patients receiving REYATAZ and PI comparator regimens. Atrioventricular (AV) conduction abnormalities were asymptomatic and generally limited to first-degree AV block. There have been rare reports of second-degree AV block and other conduction abnormalities and no reports of third-degree AV block. Caution should be used when REYATAZ is given concurrently with other drugs that prolong the PR interval (including beta-blockers, diltiazem, verapamil and digoxin), especially drugs metabolized by CYP3A or in patients who have preexisting cardiac conduction system disease.
· New-onset or exacerbation of preexisting diabetes mellitus and hyperglycemia have been reported in patients treated with protease inhibitor therapy. Increased bleeding has been reported in hemophiliacs treated with protease inhibitor therapy.
· Reversible, asymptomatic elevations in indirect (unconjugated) bilirubin occurred in most patients treated with REYATAZ. Alternative a ntiretroviral therapy to REYATAZ may be considered if jaundice or scleral icterus presents cosmetic concerns. Caution should be used with administration of REYATAZ to patients with hepatic impairment, including those with hepatitis B or C and patients with marked elevations in transaminases.
· Rash (all grades, generally mild-to-moderate maculopapular skin eruptions, regardless of causality) occurred in 21% of patients treated with REYATAZ in controlled clinical trials. REYATAZ should be discontinued if severe rash develops. Cases of Stevens-Johnson syndrome and erythema multiforme have been reported in patients receiving REYATAZ.
· Various degrees of cross-resistance among protease inhibitors have been observed.
· Redistribution and/or accumulation of body fat have been seen in patients receiving antiretroviral therapy. A causal relationship has not been established.
· Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including REYATAZ.
· Commonly reported side effects of moderate or severe intensity in adult treatment-naïve patients include: nausea (14%), jaundice/scleral icterus (7%), rash (7%), headache (6%), abdominal pain (4%), vomiting (4%), peripheral neurologic symptoms (4%), diarrhea (3%), insomnia (3%), and dizziness (2%). Commonly reported side effects of moderate or severe intensity in adult treatment -experienced patients receiving REYATAZ (atazanavir sulfate)-containing regimens include: jaundice/scleral icterus (9%), myalgia (4%), diarrhea (3%), nausea (3%), fever (2%), and depression (2%).
In therapy-naïve patients, REYATAZ 400 mg should be taken once daily with food. In therapy-experienced patients, REYATAZ 300 mg plus ritonavir 100 mg should be taken once daily with food. Efficacy and safety of REYATAZ with ritonavir in doses greater than 100 mg once daily have not been established. Prescribers should consult the complete prescribing information for Norvir (ritonavir) when using this agent.
Please refer to the enclosed REYATAZ Full Prescribing Information.
|
|
| |
| |
|
|
|