 |
 |
 |
| |
Tenofovir + FTC & Efavirenz vs Fixed Dose Combination Combivir (AZT/3TC): 24 week results, study 934
|
| |
| |
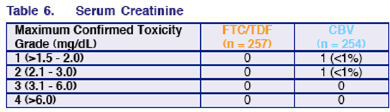
Jose Arribas presented these study results in an oral late breaker at Glasgow last week. What I think is new compared to the presentation previously at ICAAC is data on adverse events related to anemia and baseline NNRTI resistance. The once daily fixed dose combination of TDF/FTC has recently been approved. This is the first large Phase III trial comparing the individual agents to the twice daily fixed dose combination CBV. Briefly, 73% taking TDF/FTC had <50 copies/ml & 65% taking Combivir had <50 copies/ml. 87% taking FTC/TDF & 78% taking Combivir had <400 copies/ml. The difference in response rates was driven mainly by discontinuation for adverse events (9% Combivr, 3% FTC/TDF): anemia, nausea, fatigue, vomiting. No patients with viral failure by week 24 have developed K65R. There has been no serum creatinine elevations for patients taking TDF/FTC.
517 treatment-naïve patients were randomized 1:1 to receive either tenofovir+FTC+efavirenz (all once daily) or Combivir (AZT/3TC)+efavirenz, Combivir is twice daily. The Fixed Dose Combination tablet of tenofovir/FTC was not used in this study. This is a 96-week non-inferiority study with a 24 week planned interim analysis. Adequate renal & hepatic function were required at baseline. The primary endpoint is Time to Loss of Virologic Response (TLOVR); switching EFV to NVP due to CNS toxicity was not considered failure.
The patient population had advanced with average viral load of 100,000 copies/ml at baseline, 51% of patients had >100,000 copies/ml, and over 40% had less than 200 CD4s & 13% with less than 50 CD4s.
The baseline characteristics were comparable between the two arms: age: 36 yrs; 14% female; 58% white, 22% black, 15% Hispanic; HIV RNA: 100,000 copies/ml; HIV RNA >100,000 copies/ml: 51%; median CD4 count: 237; 42% <200 CD4s; 13% <50 CD4s.
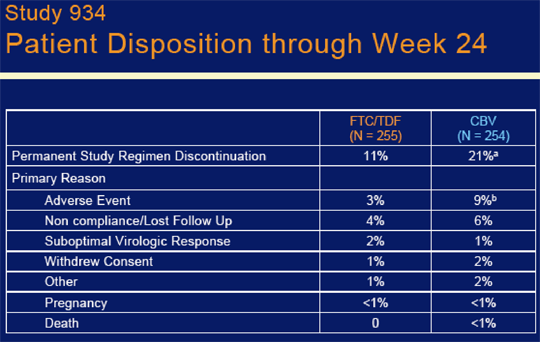
Study discontinuation was higher in the Combivir arm (21% vs 11%, p=0.003). This was driven by a higher adverse event rate in patients taking Combivir (9% vs 3%, p=0.008) who withdrew due to adverse events. Withdrawal due to suboptimal viral response was 2% in FTC/TDF arm 7 1% in CBV arm. Viral failures were .
|
|
| |
| |
 |
|
| |
| |
BASELINE NNRTI RESISTANCE
Of note, 22 patients had NNRTI drug resistance before starting the study. Apparently, they may have acquired HIV with drug resistance. 20 of the patients were from the USA & 2 from Germany. There were 11 in the TDF/FTC arm & 11 in the Combivir arm. Investigators were notified & the FDA recommended excluding these patients for week 48 primary endpoint analysis (n=487). Primary efficacy endpoint (HIV RNA <400 c/ml) at week 24 analyzed for both populations, excluding NNRTI-resistant (n=487) and ITT (n=509).
% Patients with HIV RNA <400 c/ml (TLOVR) ITT (n=509) at week 24
FTC/TDF: 87%
CBV: 78%
P=0.010
95% CI: (+1.9%, +14.9%)
% Patients with HIV RNA <50 c/ml (TLOVR) ITT (n=509) at week 24
FTC/TDF: 73%
CBV: 65%
P=0.038
95% CI: (+0.5%, +16.2%)
% Patients with HIV RNA <400 c/ml (TLOVR) Excluding NNRTI-resistance (n=487)
FTC/TDF: 88%
CBV: 80%
P=0.019
95% CI: (+0.8%, +13.3%)
CD4 increases were a mean 129 for FTC/TDF & 111 for CBV.
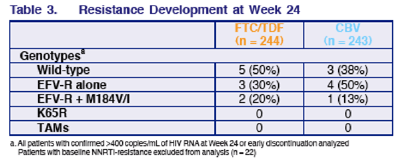
RESISTANCE DEVELOPMENT AT WEEK 24
Viral failures with >400 copies/ml at week 24 (excluding NNRTI-r at baseline)-- wild-type: 5 (50%) FTC/TDF 3 (38%); EFV-r alone: 3 (30%) FTC/TDF, 4 (50%) CBV; EFV-r+184: 2 (20%) TDF/FTC, 1 (13%) CBV; K65R: 0 & 0; TAMS: 0 & 0.
|
|
| |
| |
 |
|
| |
| |
GRADE 3-4 ADVERSE EVENTS THROUGH WEEK 24
| FTC/ADV | CBV | | # w/any AE | 24 (9%) | 38 (15%) | | AE | | | | -anemia | 0 | 10 (4%) | | -neutropenia | 1 (<1%) | 3 (1%) | | -diarrhea | 3 (1%) | 1 (<1%) | | -fatigue | 0 | 3 (1%) | | -depression | 1 (<1%) | 2 (1%) |
Grade 3 anemia: Hgb 6.0-6.9 g/dL; Grade 4 anemia: Hgb <6 g/dL.
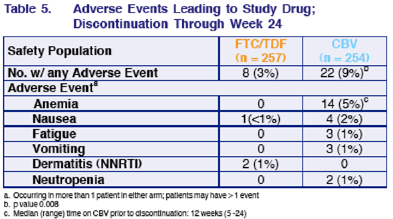
ADVERSE EVENTS LEADING TO STUDY DRUG DISCONTINUATION THROUGH WEEK 24
There were 22 (9%) patients in the CBV & 8 (3%) in the FTC/TDF arms with any AE. AEs leading to discontinuation for patients taking CBV were : anemia 14 (5%), no patients taking FTC/TDF disct due to anemia; 3 patients taking CBV disct due to fatigue & 3 due to vomiting, and 2 due to neutropenia, and 4 due to vomiting. For the 8 patients listed as discontinuing TDF/FTC due to AE (n=8, 3%), 1 was due to nausea & 2 due to NNRTI related dermatitis, that was all that was listed.
|
|
| |
| |
 |
|
| |
| |
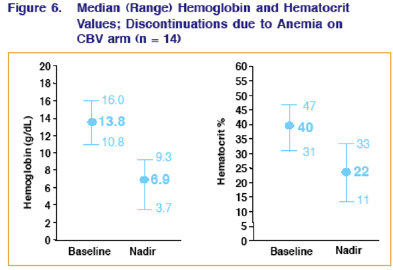
HEMOGLOBIN & HEMATOCRIT VALUES FOR PATIENTS DISCONTINUING CBV DUE TO ANEMIA
For patients who discontinued CBV due to anemia, their median baseline & nadir (lowest value) for hemoglobin was 13.8 at baseline & 6.9 at nadir; for hematocrit it was 40 at baseline & 22 at nadir.
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
|
| |
|
 |
 |
|
|