 |
 |
 |
| |
Lipoatrophy in HIV: can it be prevented & improved; Risk for Heart Disease in HIV
|
| |
| |
Written by Jules Levin
The Workshop ended today Thursday. Two important topics of discussion at the Workshop were heart disease in HIV, and the relationship between NRTIs and lipoatrophy. Of note, concern was expressed about fatty liver in HIV but no mention was made regarding the potential for coinfected (HCV or HBV+HIV) patients being at risk for double the hit for fatty liver, as fatty liver is associated with lipid elevation, insulin resistance, and having hepatitis. Fatty liver can increase risk for liver cancer and reduce response to HCV therapy PegIFN/RBV.
Before attending the Workshop I had decided that the risk for heart disease is not receiving adequate attention by the HIV medical community and researchers, and that the risk for developing heart disease is generally underestimated by patients, providers, and researchers. Several studies and discussions presented at the Workshop emphasize there is a risk. In particular, a plenary talk by Enzo Bonora from Italy delivered this message to the audience. After his talk I went to the microphone and expressed my serious concerns and encouraging more attention to the problem.
Here is a brief summary of the talk by Bonora, who is an endocrinologist and discussed findings from his studies in Italy of 1000 people ages 40-79 from 1990-95. Diagnostic criteria for Metabolic Syndrome include: impaired glucose regulation (IFG, IGT, or DM) or insulin resistance, and additional factors: dyslipidemia (TG>150) and/or HDL <35 men, <39 women), hypertension (>=140/90 mmHg), obesity (BMI>30) and/or central fat distribution (WHR >0.9 men, >0.85 women), and microalbuminuria (ACR>=30 mg/g); fasting glucose >110; HDL <40 men, <50 women. In his study in HIV-negatives, the prevalence was high for features of Metabolic Syndrome: 70% central obesity, 50% IFG/GT/DM, 30% dyslipidemia, hypertension 60%. 70% had 2 or more features or disorders. Men & women had disorders equally. TG, HbA1c, HDL-C, insulin resistance, central obesity, dyslipidemia, mild hypertension, microalbuminemia, and IFG/GT were associated with risk for developing heart disease. The Metabolic Syndrome is extremely common among mature-advanced age individuals. Insulin resistance is a common among features of this Syndrome. Individuals with the Metabolic Syndrome have an increased risk for heart disease
As well, several studies presented at the Workshop addressed NRTIs and lipoatrophy. Much research has suggested that mitochondrial depletion is caused by certain NRTIs and is associated with lipoatrophy. David Nolan gave an oral talk presenting results from a study in which patients on various NRTIs received biopsies. From his research and from other research he concluded that abacavir and tenofovir don't lead to mitochondrial depletion and don't cause lipoatrophy. Researchers from Greece presented 3 interesting posters on body composition changes. Two of the posters report on studies of a small number of patients who switched the NRTI-component of their therapy to a tenofovir-based regimen and saw improvements in body composition as observed by objective measures. The third Greek study followed patients for 10 years with objective tests and reported a considerable amount of limb fat lost. There were two posters at the Workshop reporting on the proportion of study participants who developed body changes in the SOLO study. This study compared treatment-naïve patients receiving fosamprenavir/ritonavir once daily to nelfinavir twice daily. Body composition changes were evaluated by physician observation, weight, and waist-to-hip ratios at three visits (baseline, week 24, week 48). Of note the proportion of patients reporting lipoatrophy was only 3%. This could be due to the fact that the NRTI regimen in the study was abacavir/3TC.
In poster 46, Greek researchers report on 17 treatment-experienced patients (11 men, 8 females). The ART regimen was switched to tenofovir+NRTI plus a PI or NNRTI. They don't report which drugs patients were previously taking. This is a small study and patients appear to be selected. Patients' body composition was assessed at baseline and week 48 by DEXA. There were no significant changes in body weight, lean body mass and whole body bone mineral content between the two assessments. Total fat tissue mass significantly increased by 13% from 16.5 kg to 18.8 kg. Total fat increased from 22.7 kg to 24.60 kg. There was a significant increase in both limb fat (15%) and trunk fat (13%). Arm fat increased from 1.7 to 2.01 kg (16%); legs fat increased from 3.6 to 4.2 kg (16%); limb fat increased from 5.3 to 6.2 kg (15%); trunk fat increased from 10.7 to 12.1 kg. But the baseline fat was so low due to prior fat loss that the increases did not approach normal fat levels. As has been seen in other studies lipid improvements occurred: cholesterol decreased by 9% from 240 to 217 mg/dL; HDL increased by 6.8% from 44 to 47; LDL decreased from 140 to 126 (NS), and triglycerides decreased from 316 to 235 (NS).
In poster 47, 10 treatment-experienced patients with lipodystrophy who were taking d4T regimen replaced d4T with tenofovir without switching the other drugs. Again, this was a small study where you could also say patients were selected. Patients were assessed by DEXA at baseline & week 52. Patients experienced increase in body weight from 68 to 71.7 kg. Bone mineral content remained stable. Lean body mass was not affected. Total fat tissue mass significantly increased by 30% from 12.4 to 16 kg. BMI significantly improved from 22.56 to 23.71 kg/m2. Significant gains in fat occurred in the trunk (28%) and in the arms (41%), but not in the legs (+33%). No significant changes in cholesterol or triglycerides were noticed. Arms fat (1.2 to 1.7 kg), legs fat (2.2 to 2.9 kg), limb fat (3.4 to 4.6 kg), trunk fat (8.5 to 11 kg) significantly improved from 28% to 41%. TG declined from 360 to 247 (NS) & cholesterol from 246 to 223 (NS).
In contrast to the two studies above, the same Greek researchers followed 21 patients for 10 years to determine body composition changes (poster 48). DEXA was performed at baseline in 1994 and in 2004. Patients exhibited significant decrease in body weight (-7.7%). Weight loss was exclusively due to fat loss: in the legs (-62%) and the arms (-52%). Trunk fat did not change significantly. Trunk/limb fat ratio exhibited significant increase. Researchers reported that peripheral fat loss was evident in patients with lipoatrophy and as well with central fat accumulation.
Of note, baseline fat was much higher for these patients in 1994 than they were for the treatment-experienced patients in the 2 studies above. So increases in fat in these two studies above did not approach the baseline levels in this study. Fat total: 19.6 baseline vs 14.1 kg followup (-26%); arms fat: 2.7 vs 1.3 kg (-50%); legs fat: 6.9 to 2.6 kg (-62%); limb fat: 9.6 to 3.6 kg (-60%). For the 9 patients with lipoatrophy at followup decreases from baseline to followup assessment were of greater magnitude: -43% total fat from 21 to 12 kg; -60% arms fat from 3 to 1.2 kg; -75% legs fat from 7.8 to 1.9 kg; -70% limb fat from 10.9 to 3.1 kg. Cholesterol increased from 175 to 218 & TG from 138 to 274.
SOLO was a randomized study in 600+ ART-naïve patients comparing fosamprenavir/ritonavir once daily to nelfinavir twice daily. These are the first prospective body composition changes data from a controlled clinical trial for FPV/r. Of note, both low baseline CD4 count (<200) & high viral load (>100,000) was associated with a higher proportion of subjects reporting fat wasting at baseline. The proportions of patients in both the high VL & low CD4 groups who reported fat wasting decreased at week 24 & then again at week 48. All patients received abacavir+3TC as the NRTI component of the regimen. Baseline CD4 counts were low: median of 170. Body composition changes were assessed by physician observation and WHR. Of patients reporting no fat wasting at baseline, and who had an assessment at week 48, only 4% (9/215 and 9/232) reported fat wasting at week 48 in both groups (FPV/r & NFV). And with followup to week 120 for patients taking FPV/r: 5% (8/161). Of patients who did not report fat accumulation at baseline and had an assessment at week 48, 15% (33/219) and 18% (44/238) reported signs of fat accumulation at week 48 in the FPV/r and NFV groups, respectively. Looking at gender: at week 48, the proportions of fat wasting were low & similar between the men & women. However, 32% (43/133) of females reported fat accumulation compared to 15% (54/355) of men, at week 48. The same trend was observed at week 120 for females. By race: at week 48, proportions with fat wasting were low & similar between blacks & whites. Both groups reported increases in fat accumulation over time & at week 48 the proportion of blacks reporting fat accumulation was greater than among whites (27% vs 15%; 7% & 5% at baseline). The proportion of patients reporting fat accumulation did not increase much (19%) at week 120.
PREVENTING & IMPROVING LIPOATROPHY
"Differential Effects of Nucleoside Reverse Transcriptase Inhibitor (NRTI) Regimens on Adipocyte (fat cells) Mitochondrial DNA Depletion in HIV-infected Patients"
SUMMARY: In this study, David Nolan reported data suggesting that patients who started with abacavir or tenofovir don't experience mtDNA loss in fat cells. Patients who started with AZT or d4T did experience mtDNA loss, but this improved after switching to tenofovir or abacavir. Nolan concludes that you can minimize or prevent lipoatrophy by starting therapy with tenofovir or abacavir. And if you switch from AZT or d4T to abacavir or tenofovir you can improve mtDNA depletion. His research and clinical experience, and the MITOX & TARHEEL studies find that switching from AZT/d4T to TDF/ABC improves mtDNA and lipoatrophy. But in the MITOX & TARHEEL studies many patients did not observe improvement merely by their observation. Nolan feels that after switching nukes the cell death (apoptosis of fat cells) stops & mtDNA depletion returns to normal but lipoatrophic damages although improving remain damaged. Tissue is damaged so the question is: can you develop new fat cells, can you recover from lipoatrophy? Nolan believes you can generate new fat cells. In healthy adults new fat cells can be generated. But can a lipoatrophic person regenerate fat tissue to a normal level, how much of this damage can be reversed? In his patient populations patients are recovering fat but will they recover fat back to normal levels? Improvement continues in his patients and the improvement is not plateauing. So, evidence is that recovery continues. But, it's a process that may take years. How many years, we don't know. But the question remains will they recover back to normal? Follow-up will tell.
COMMENTS from Jules Levin: people commencing treatment can perhaps avoid lipoatrophy by selecting NRTIs properly. After 120 weeks 3% of patients in the SOLO Study developed fat loss. And in the Gilead 903 Study 3% of patients taking TDF/3TC/EFV developed fat loss.
Nolan and colleagues from Perth, Australia first showed prior study results from three studies reporting fat wasting in 12-19% of patients taking AZT/3TC and in 50% of patients taking d4T/3TC or d4T/ddI. He provided a model of NRTI induced lipoatrophy. mtDNA depletion can occur within the first 3-12 months on d4T, perhaps delayed on AZT, and clinical lipoatrophy can occur in 10-40 months. Clinical lipoatrophy can result in leg fat of 24% or as little as 11% of the original fat mass. In NRTI naïve patients the amount of mtDNA in a fat cell can be 1622 copies/cell; in a patient on AZT it can be 537 copies/cell, and on d4T it can be 234 copies/ml (Nolan D et al, Antiv Ther 2003; 8:617-28).
In the study he presented at the Workshop there were 103 individuals and 147 biopsies: HIV- controls (n=7); ART naïve HIV-infected (n=34); current AZT therapy (n=41); current d4T therapy (n=35); non-thymidine analogue therapy (abacavir, tenofovir) (n=25); no current therapy (n=7). This is an ongoing non-randomized cohort study. Biopsy procedure: excisional biopsy subcutaneous fat (3-5 cm incision), suprailiac site. Measurement: adipocyte mitochondrial DNA and adipose tissue (copies/cell) and RNA content determined using quantitative real-time PCR (ABI Prism 7700).
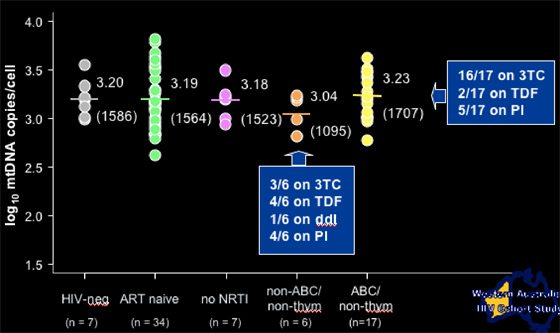
Each study patient group received 25-43 biopsies. Most patients were taking 3TC. About 40% were taking a PI. Average age was 45 yrs. Average CD4 count was 600+ for patients receiving AZT, d4T, or non-thymidine drugs, and 340 for HIV-infected controls. Months on drug was 37 for AZT, 31 for d4T, and 11 for non-thymidine. There were no differences in these baseline characteristics between the patient groups.
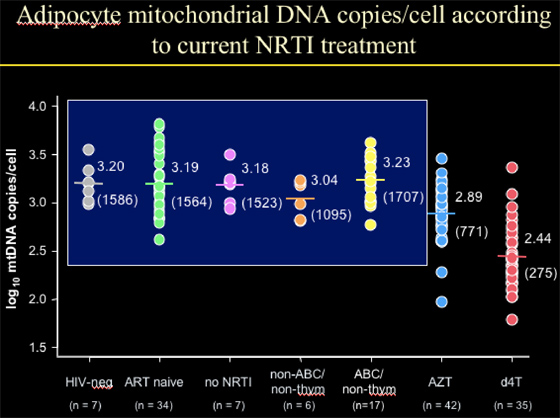
Adipocyte mitochondrial DNA copies/cell according to current NRTI treatment
NIV-neg: 1586 copies/cell (n=7)
ART-naïve: 1564 copies/cell (n=34)
No current therapy: 1523 copies/cell (n=7)
--The point is that HIV+ individuals, HIV+ not on current therapy, and HIV-negative patients had the same number of mtDNA copies/cell.
Age, viral load, BMI, & CD4% were not associated with mtDNA levels in fat cells (adipocytes).
--for the 35 patients on d4T, they had 275 mt DNA copies/cell, which is 18% of the values for the groups immediately above. This translates into a decline of 82%.
--for patients on AZT (n=42), they had 771 copies/cell, which is 49% of the values for the 3 groups above. This translates into a decline of 51% (d4t vs AZT decline, p<0.0001.
--for patients on abacavir, they had 1707 copies/cell (n=17)
--for patients on tenofovir, they had 1095 copies/cell (n=6, 1 pt was on ddI, 4/6 on TDF).
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
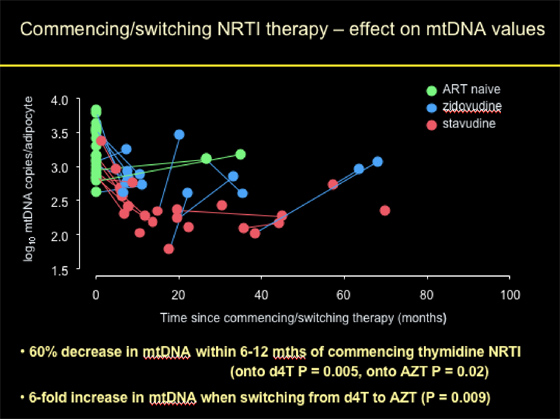
Commencing/switching NRTI Therapy: effect on mtDNA Values
Patients who were ART-naïve had normal mtDNA copies/adipocyte at baseline. Patients who initiated d4T or AZT (thymidine NRTI) experienced on average a 60% decrease in mtDNA within 6-12 months of commencing therapy (onto d4T p=0.005), onto AZT p=0.02). Patients who started with d4T appeared to have experienced worse declines (although difference not significant) in mtDNA compared to patients starting with AZT. Patients who switched from d4T to AZT experienced a 6-fold increase in mtDNA when switching (p=0.009).
As you can observe from the graph/pic: "% decrease and fold increase can be said to be median (mean on log scale) -- in value AZT is actually more than d4T, but not significantly different and AZTs have much more variability and start higher so have combined the 2."
|
|
| |
| |
 |
|
| |
| |
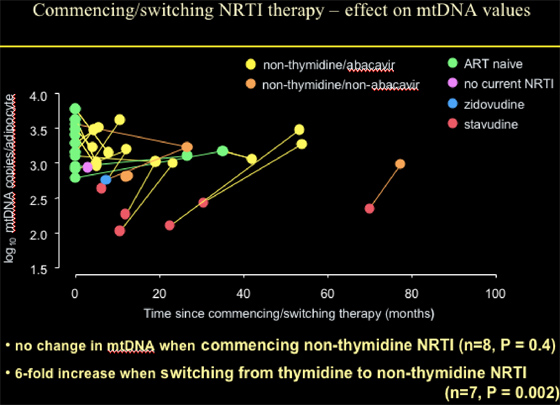
Patients commencing with or switching NRTI therapy to abacavir or non-thymidine/non-abacavir (mostly TDF) showed no change in mtDNA when commencing non-thymidine NRTI (n=8, p=0.4); or a 6-fold increase when switching from thymidine to non-thymidine NRTI (n=7, p=0.002).
|
|
| |
| |
 |
|
| |
| |
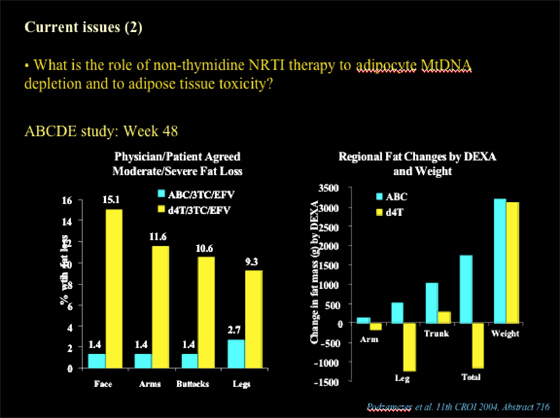
Nolan showed this slide below of data presented at CROI 2004 (abstract 716) from the ABCDE Study. At week 48, 1.4-2.7% of patients who received ABC/3TC/EFV experienced peripheral fat loss and 9-15% of patients who received d4T/3TC/EFV had peripheral fat loss (face, arms, buttocks, legs), as evaluated by physician & patient (moderate/severe fat loss). Using DEXA to evaluate regional fat changes (arms, leg, trunk, total fat, and also total weight was reported: patients receiving ABC & d4T had weight gain, but d4T patients had total fat loss & in these regions, while ABC patients had increased fat mass in these regions & in total fat.
|
|
| |
| |
 |
|
| |
| |
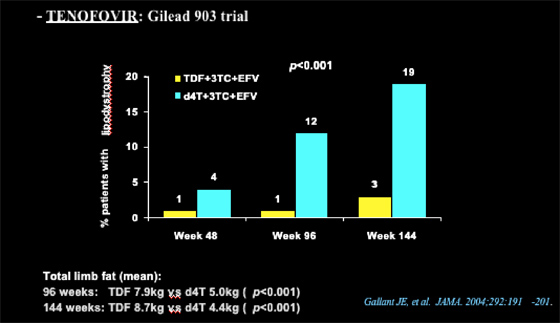
Nolan referred to the Gilead tenofovir 903 study, which showed that 3% of patients receiving TDF/3TC/EFV & 19% receiving d4T/3TC/EFV developed lipodystrophy at week 144 (12% vs 1% at week 96, 4% vs 1% at week 48, respectively).
Total limb fat
at 96 weeks: TDF 7.9 kg vs d4T 5.0 kg (p<0.001);
at 144 weeks: TDF 8.7 kg vs d4T 4.4 kg (p<0.001)
|
|
| |
| |
 |
|
| |
| |
|
| |
|
 |
 |
|
|