| |
New national liver transplant allocation policy: Is the regional review board process fair?
|
| |
| |
Michael D. Voigt 1 *, Bridget Zimmerman 2, Daniel A. Katz 3, Stephen C. Rayhill 3
1Department of Internal Medicine, Roy A. and Lucille J. Carver College of Medicine, University of Iowa, Iowa City, IA
2Department of Biostatistics, Roy A. and Lucille J. Carver College of Medicine, University of Iowa, Iowa City, IA
3Department of Surgery, Roy A. and Lucille J. Carver College of Medicine, University of Iowa, Iowa City, IA
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and do not represent official policy of or interpretation by the OPTN or the U.S. Government.
Abstract
Experienced transplant professionals may predict mortality better, in highly selected cirrhotic patients referred for accelerated listing to regional review boards, than the (Pediatric) Model for End-Stage Liver Disease score. However, these requests are often denied. We wished to establish if (1) such denials increase mortality and (2) referring physicians predict mortality better than the score. We analyzed 1,965 non-status 1 requests made between February and November 2002 from the United Network for Organ Sharing (UNOS) national scientific registry. Kaplan-Meier survival and time to transplant were compared between denied and approved patients. Cox proportional hazards analysis was used to establish if referring physicians predicted mortality better than the score. More requests were denied for patients with nonsanctioned conditions (45.7%) than for those with sanctioned conditions (13.3%); P less than .0001). Fewer patients denied accelerated listing had a transplant (46.6% vs. 63.8%; P < .0001); time to transplant was similar (P = .2).
However, nonsanctioned cirrhotic cases denied accelerated listing had lower mortality than approved cases (P < .04). Referring physicians predict mortality poorly (P = .23), whereas the Model for End-Stage Liver Disease (MELD)-Pediatric Model for End-Stage Liver Disease (PELD) score was highly predictive (P = .0003). In conclusion, regional review boards are fair and can accurately distinguish high- from low-risk patients. Denying requests does not increase mortality. The MELD-PELD score remains the best predictor of mortality, but the review board process adds additional information. Referring physicians predict patient mortality poorly. (Liver Transpl 2004;10:666-674)
Article Text
There are insufficient donors for the ever-increasing number of patients listed for liver transplantation. The United Network for Organ Sharing (UNOS) policy changed in February 2002, so that patients at highest risk of short-term, pretransplant mortality receive priority for cadaver liver allocation. The policy mandates that the Model for End-Stage Liver Disease (MELD) score for adults[1] and the Pediatric End-Stage Liver Disease (PELD) score[2] for pediatric patients (MELD-PELD score [MPS]) be used as the primary ranking for patients listed for cadaver liver transplantation.[3] The policy change was made possible because the MPS was the first well-validated, objective measure of short-term mortality.[4]
The MELD score is calculated using serum creatinine, bilirubin, and International Normalized (Prothrombin) Ratio.[1] The PELD score is similar but incorporates patient age, albumin, and growth failure into the equation.[2]
The MELD and PELD scores have been well validated in several large studies to accurately predict short-term mortality.[1][2] The presence of complications had minimal effect on its predictive accuracy.[1][5-9]
The new UNOS policy specifies that cadaver livers be allocated to the patient on the list with the highest MELD or PELD score. This has been an important advance toward an objective evidence-based approach to organ allocation.[4]
However, in some diseases that liver transplantation can cure, the MPS does not accurately predict the mortality risk. UNOS rules exempt patients with these sanctioned conditions from using their true MPS to determine priority on the waiting list and provide guidelines for the magnitude of the increase in MPS. Patients are listed with the increased score if diagnostic and staging criteria for those conditions are met, and regional review boards (RRBs) consider the request appropriate.[3] The additional MPS points move the patients up the liver waiting list and hence reduce their waiting time.
These conditions and their criteria are specified in policy 3.6: policy 3.6.4.4- (hepatocellular cancer); policy 3.6.4.4.1(hepatoblastoma); policy 3.6.4.5.1(hepatopulmonary syndrome); policy 3.6.4.3 (metabolic diseases); and policy 3.6.4.5.2 -(primary oxaluria and familial amyloidosis).[3]
Alternatively, physicians may request accelerated listing for patients that they believe have worse mortality than predicted by the MPS in conditions not sanctioned by UNOS (e.g., recurrent biliary sepsis in patients with primary sclerosing cholangitis). UNOS does not provide guidelines for the magnitude of the increase in score in these nonsanctioned cases. The referring physician requests an increase in score based on his or her perception of the patient's mortality risk. RRBs only approve listing with the increased score in cases they judge appropriate. If RRBs deny the request, patients remain listed with their calculated (true) MPS. Every UNOS region has an RRB made up of a representative from each liver transplant center in that region.
For UNOS-sanctioned conditions, the RRB process is objective and consistent. However, it is subjective, and therefore potentially inconsistent, for requests in patients with nonsanctioned conditions. In addition, experienced transplant physicians intuitively may be able to predict mortality better than the MELD or PELD score in these highly selected patients with nonsanctioned conditions. If this were true, patients denied the increased score would have higher mortality. The predictive accuracy of MELD and PELD scores has not been specifically evaluated in these highly selected cases. Hence, we do not know if nonsanctioned cases denied accelerated listing under the new UNOS policy are at higher risk of dying. The change in policy has therefore raised the important question of whether the RRB process is fair.
To address these questions, we performed this study to (1) establish if subjects denied accelerated listing by RRBs have increased mortality before receiving a transplant compared to those approved for accelerated listing and (2) establish if the referring physician's assessment of mortality risk is superior the MELD-PELD score in the highly selected patients referred to RRBs with nonsanctioned conditions.
Abbreviations:
UNOS, United Network for Organ Sharing; MELD, Model for End-Stage Liver Disease; PELD, Pediatric End-Stage Liver Disease; MPS, MELD-PELD score; RRB, regional review board; OR, odds ratio; HR, hazard ratio.
Patients and Methods
To determine death rates in patients referred to RRBs, we analyzed data of all nationwide referrals to UNOS RRBs from the inception, in February 2002, of modified UNOS policy 3.6 for liver allocation, until October 2002. Data was obtained from the UNOS National Scientific Registry. For cases with multiple requests, we used only the final RRB determination of approved or denied. Status 1 patients were excluded because they do not fall under the MPS system.
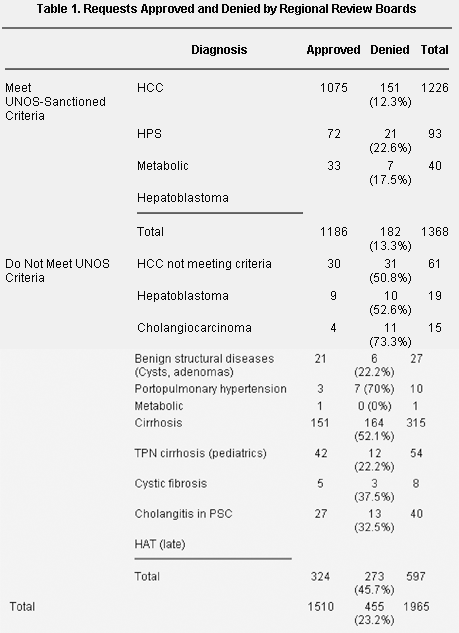
All case diagnoses are given in Table 1 and are divided into the UNOS-sanctioned and nonsanctioned categories. Pediatric patients (<18 years) were analyzed separately because cirrhotic pediatric patients in intensive care units are frequently prioritized as status 1, thereby bypassing the PELD system, and pediatric patients may be handled differently by RRBs.
|
|
| |
| |
 |
|
| |
| |
NOTE. Number of cases and percentage of cases denied are shown.
Abbreviations: HCC; hepatocellular carcinoma; TPN, total parenteral nutrition; PSC, primary sclerosing cholangitis; HAT, hepatic artery thrombosis; HPS, hepatopulmonary syndrome; UNOS, United Network for Organ Sharing.
The primary endpoint was death while awaiting transplantation. We used Kaplan-Meier analysis to compare survival of patients approved for (versus patients denied) accelerated listing. Where subjects were removed from the UNOS transplant list because the patient had become too sick for transplant, we assumed they had died. Conversely, we assumed subjects were alive if they were removed from the list because of clinical improvement. Censoring occurred at the time of removal from the list. We did Cox proportional hazard regression modeling to control for differences in baseline MPS and in requested MPS of patients approved or denied accelerated listing. We also analyzed the difference in time to transplant, using Kaplan-Meier analysis in approved and denied patients.
Analyses of mortality on the waiting list and waiting time to transplant were also done only on cirrhotic patients - with other categories excluded - because death on the waiting list is inaccurate for patients in other categories in which death does not occur due to intrinsic liver disease.
Our second main objective was to establish if referring physicians predicted mortality better than the MPS. Because the referring physicians chose the increase in MPS for their patients, the requested score indicated the risk they attributed to the patient. We did Cox proportional hazards analysis including the requested score and the baseline (true) MELD or PELD score in the model to establish if the requested score predicted mortality better than the baseline score. We repeated this analysis after categorizing cirrhosis cases separately from all other diagnoses because MPS predicts death from liver disease and is therefore relevant only in cirrhotic patients. In all other cases, including sanctioned and nonsanctioned categories (e.g., stage 3 or 4 cancer, portopulmonary hypertension), death on the waiting list does not result from liver failure. Rather, these patients become unable to undergo transplantation or have increased posttransplant mortality; hence, the MPS is not relevant in predicting mortality on the waiting list.
Finally, we analyzed whether the magnitude of the increase in MPS over the baseline score (delta-MPS) in patients referred with nonsanctioned conditions affected survival. We reasoned that a small increase would shorten wait time on the list less than a large increase, which in turn might affect outcome. We analyzed adult and pediatric patients separately. This analysis excluded cancer patients for the reasons given. A separate analysis of only cirrhotic patients was also done for the reasons given. Primary analysis was by Cox proportional hazard modeling, including baseline MPS and delta-MPS effects on survival. We also used Kaplan-Meier log-rank analysis to evaluate the effects of various delta-MPS scores on survival and on time to transplant (survival without a transplant).
Statistical analysis was done using SAS (SAS Institute Inc, Cary, NC) and SPSS (SPSS Inc, Chicago, IL) software.
Results
Description and Classification of Cases
Analysis was performed on 1,965 unique requests to RRBs for accelerated listing. This excluded status 1 patients. The majority of RRB referrals were adult (n = 1794, 91.3%) with only 171 (8.7%) pediatric referrals. The denial rate for all cases was 23% and was similar for adult (23.2%) and pediatric cases (22.8%). The number of requests and the approval rate in each group are given in Table 1 and the reasons for removal from the transplant list in Table 2. Only 13.3% of sanctioned cases versus 45.7% of nonsanctioned cases were denied accelerated listing. Odds ratio (OR) for denial of sanctioned versus nonsanctioned cases was 0.182 (95% CI, 0.146-0.228; P < .0001).
|
|
| |
| |
 |
|
| |
| |
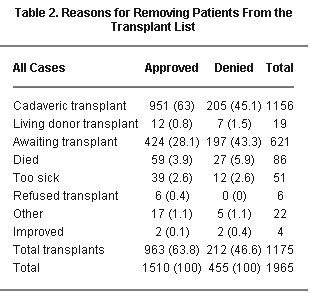
A high percentage of patients (46.6%) denied accelerated listing did receive a transplant. In cases where the RRB approved the accelerated listing, a significantly higher percentage received a transplant (63.8%, P < .0001).
The odds of a request being denied when the diagnosis was cirrhosis were 4.59 times (95% CI, 3.62-5.8) the odds for the diagnosis of malignancy (P < .0001). The OR for denial of request for cirrhosis compared to metabolic/structural diseases was 3.30, (CI, 1.8-6.1; P = .0001), and 2.2 (CI, 1.4-3.5; P = .0012) for cirrhosis compared to pulmonary complications. Pulmonary complication also had greater odds of denial than malignancy (OR 2.11; CI, 1.3-3.3; P = .001).
In the pediatric group, denial rates were 7 of 25 (28%; CI, 12.1%-49.4%) for malignancy; 3 of 10 (30%; CI, 6.7%-65.3%) for pulmonary complications; 3 of 26 (11.5%; 2.5%-30.2%) for metabolic/structural diseases; and 26 of 110 (23.6%; CI, 16.1%-32.7%) for cirrhosis (chi-square P = .458).
Comparison of Mortality Rates: Cases Meeting UNOS-Sanctioned Criteria Versus Non Sanctioned Cases
Results of approval, denial, and mortality rates of cases meeting UNOS-sanctioned criteria, compared to those not meeting criteria (nonsanctioned) cases, are shown in Table 3.
|
|
| |
| |
 |
|
| |
| |
NOTE. Sanctioned conditions are those with established criteria specified by UNOS to receive automatic accelerated listing. They receive a score that is specified by the rules. Sanctioned conditions include Stage 1 and 2 hepatocellular cancer, hepatoblastoma, hepatopulmonary syndrome, and metabolic liver disease. Nonsanctioned conditions are those that do not meet criteria specified by the UNOS rules for automatic accelerated listing. Approved cases are those granted accelerated listing (the requested increase in MELD or PELD score) by RRBs. Denied cases are those denied accelerated listing by RRBs.
Overall mortality (approved or denied) in sanctioned cases was 5.1 % (70 cases out of 1368) but was 12.2% (73 cases out of 597) in nonsanctioned cases. OR for mortality in nonsanctioned cases versus sanctioned cases was 2.24 (CI, 1.58-3.18; P < .0001).
Comparison of Mortality Rates: Cases Approved for Accelerated Listing Versus Cases Denied
We did this analysis to establish if cases that were denied accelerated listing had a higher mortality than those that were approved.
There was no significant difference in survival to transplantation with approval or denial of accelerated listing among adult (P = .91) or pediatric cases (P = .47).
Subgroup analysis showed there was no significant difference in survival to transplantation for approved or denied cases in adult or pediatric cancer (P = .77), pulmonary cases (P = .85), or cases with metabolic or structural disease (P = .57).
Non-sanctioned cases approved for accelerated listing had a higher mortality rate (48 out of 324; 14.8%) than those denied (25 out of 273; 9.6%). The OR for mortality in approved cases relative to denied cases was 2.07 (95% CI, 1.17-3.66; P < .008). This was confirmed by the Kaplan-Meier analysis that compared the survival distribution between those approved and those denied accelerated listing in all nonsanctioned cases. In adults, there was a significantly increased mortality in nonsanctioned cases that were approved for accelerated listing (P = .0003 log-rank test). The subset of nonsanctioned cases comprising only the adult cirrhotic group similarly showed significantly worse survival if approved for accelerated listing than if denied (P < .05). Similarly, after exclusion of cancer diagnoses, nonsanctioned adult cases approved for accelerated listing showed a trend toward worse survival (P = .056 log-rank). There was no significant difference in pediatric cases between those approved or denied (P = .245).
As expected, there was no significant difference in survival to transplantation in sanctioned cases between those denied or approved (P = .58); these cases typically do not die from liver failure while awaiting transplant but become unable to undergo transplantation or have posttransplant mortality.
Cox proportional hazard modeling confirmed a trend toward reduced mortality in patients denied accelerated listing compared to those approved, after adjusting for the baseline MELD or PELD score (hazard ratio [HR] 0.61; CI, 0.37-1.01, P = .055).
Effect of Approval Versus Denial on Time to Transplant
To evaluate the finding that more deaths occurred in the approved than in the denied cases in patients with nonsanctioned conditions, we analyzed the effect that the approval had on time to transplant.
In adult cirrhotic patients, time to transplant was similar in those approved or denied:time to transplant for those approved was 55 days (SE 5; CI, 45-66); for those denied, time to transplant was 48 days (SE 6; CI, 39-59); P = .219). In pediatric cirrhotic patients, time to transplant for those approved was 46 days (SE 6;CI, 34-57) versus 37 days for those denied (SE 7; CI, 23-52; P = 0.53). Analysis of all cases with nonsanctioned conditions and, separately, of pulmonary and metabolic groups - for adult or pediatric patients - also showed no significant difference in time to survival between cases approved versus those denied (data not shown).
Therefore, the increased mortality in approved cases is not explained by increased time to transplant.
Effect of the Magnitude of MPS Increase (Delta-MPS) on Survival and Time to Transplant
To evaluate the effect of the magnitude of the increase in score (delta-MPS) on survival, Cox proportional hazard analysis was performed with the baseline and delta-MPS as independent variables in the model. This showed there was a small independent effect of the delta-MPS on mortality. The magnitude of the delta-MPS was inversely proportional to the risk of death while awaiting transplant in noncancer, nonsanctioned cases. (Parameter estimate -0.00673; HR 0.99; CI, 0.987-0.999; P = .02). However, the predominant effect on mortality in this model was due to the baseline MPS (HR 1.47; CI, 1.20-1.80; P = .0003).
Patients with nonsanctioned diagnoses referred with delta-MPS greater than or equal to 15 had significantly better duration of survival (Kaplan-Meier log-Rank analysis) than those with less than 15 additional points (mean survival time 256 days; SE 5; CI, 46-266 vs. mean survival time 216 days; SE 8; CI, 200, 232; P < .00001). In cirrhotic patients (nonsanctioned cases excluding cancer, pulmonary, and metabolic and structural liver disease categories), survival was also significantly better if delta-MPS was greater than 15 points (mean survival time 252 days; SE 7; CI, 239-265 vs. mean survival time 195 days; SE 9; CI, 178-252; P = .0001).
This held true for adult cirrhotic patients (P = .0005) and for pediatric cirrhotic patients (P = .015).
Furthermore, in adult cases with nonsanctioned conditions, better survival with a delta-MELD greater than or equal to 15 points (P < .04) was only seen in approved cases, not in cases denied accelerated listing. Similarly, pediatric cases with delta-PELD greater than or equal to 15 points had better survival only if approved (P = .02), not denied. (P = .24).
Thus, there was significantly improved survival in approved nonsanctioned patients with larger delta-MPS. To evaluate this further, we analyzed whether there were differences in baseline MPS and whether patients with large delta-MPS had shorter waiting times to transplant.
The baseline MPS in the group with delta-MPS less than 15 (18.8; SE 6.2; CI, 18.1-19.5) was strikingly higher than those with delta-MPS greater than or equal to 15 (12.0; SE 5.1; CI, 11.2-12.8; P < .0001). Thus, there was a systematic tendency for referring physicians to request a bigger increase in score for those with low baseline MPS and a smaller increase in score for those with high baseline MPS.
The high delta-MPS was associated with a significantly reduced time to transplant (mean 37 days; SE 4; CI, 30-44) compared to low delta-MPS (mean 57 days; SE 5; CI, 48-67;) p=0.001) for all cirrhotic patients (adult and pediatric) and for adult cirrhotic patients (mean 36 days; SE 5; CI, 26-46 vs. mean 57 days; SE 5; CI, 48-67; P = .0001).
This reduction in time to transplant in adult cirrhotic patients was only observed if patients were approved: 26 days (SE 3; CI, 19-32) versus 57 days (SE 7; CI, 42-72; P = .002). Reduction in time to transplant in adult cirrhotic patients was not observed in those denied: 48 days (SE 10; CI, 28-68) versus 58 days (SE 6; CI, 45-71; P = .37). Similarly, pediatric cases approved with greater than or equal to 15 points had shortened time to transplant: 26 days (SE 6; CI, 14-39) versus 60 days (SE 10; CI, 41-78; P = .02). However, there was no difference in denied cases: 33 days (SE 8; CI, 18-48) versus 31 days (SE 11; CI, 9-53; P = 0.8).
Predictive Accuracy of the MELD or PELD Score Compared to Referring Physicians' Assessment of Mortality Risk
For all cases, Cox proportional hazards modeling revealed that only the baseline (laboratory) MPS significantly predicted the risk of death while awaiting transplantation (P = .0003). The requested MPS (P = .23) and the delta-MPS (P = .08) did not have a significant contribution to the model after accounting for the effect of baseline MPS.
Using Cox proportional hazard regression in nonsanctioned cases, we showed that true (laboratory) MPS score had the strongest positive association with mortality in patients awaiting transplantation: HR 1.11; CI, 1.07-1.16; P < .0001). The delta-MPS showed a weaker negative association with risk of death while awaiting transplant (HR 0.95; CI, 0.89-1.01; P = .093); this also corresponds to the association of the requested MPS with risk of death, accounting for baseline MPS score.
Because the MPS specifically predicts death from liver failure, it strictly applies only to patients referred to RRBs with cirrhosis. Patients in other categories, whether sanctioned (e.g., hepatopulmonary syndrome, stage 1 or 2 hepatocellular cancer) or not sanctioned (e.g., portal pulmonary hypertension, stage 3 or 4 hepatocellular cancer), do not die from liver failure but become unable to undergo transplantation or have increased posttransplant mortality. As a result, we also did Cox proportional hazards analysis for cirrhotic patients versus those with all other diagnoses. Again, this showed that only the laboratory (true) MPS had predictive value (HR 1.14; CI, 1.11-1.175; P < .0001) and that the requested score had no predictive value (HR 0.98; CI, 0.94-1.02; P = .33).
As expected, there was no significant association between baseline MPS and mortality while awaiting transplant in cancer patients (P = .3). There was also no association between the requested MPS (P = .67) or the delta-MPS (P = .58) in the cancer group or in the other sanctioned categories (data not shown). This is expected because mortality in hepatocellular cancer and other sanctioned cases by definition does not depend on the severity of the liver disease.
Discussion
Neither the MELD nor PELD score has been specifically tested in the highly selected subgroup of very ill patients referred to RRBs. Experienced transplant physicians may be able to predict short-term mortality of this subgroup better than the MPS. If this were true, the cases denied accelerated listing would have increased mortality. This study was done to assess the fairness of the new UNOS policy, to establish if mortality was increased in cases denied accelerated listing, and to establish if experienced physicians predict mortality better than the MELD or PELD score.
The most important finding of our study was that survival in cases denied accelerated listing was not worse than in those approved for accelerated listing. Indeed, adults with cirrhosis and other nonsanctioned conditions who were denied accelerated listing by RRBs actually had a reduced mortality compared to those approved for accelerated listing (P < .0003). Reduced mortality in denied cases was not seen in pediatric cases (P = .245), most likely because of the small number of patients and because many of the sickest cirrhotic pediatric patients are listed as status 1 and therefore bypass the PELD system.
This finding shows that the RRB process is functioning as it is supposed to. Denials are not jeopardizing patient lives. In nonsanctioned cases, RRBs are able to identify the sickest patients and grant approval, while denying accelerated listing to low-risk cases. RRBs appear to be providing an objective and fair assessment of cases and are able to distinguish high- from low-risk candidates.
The reduced mortality in denied cases is not explained by differences in time to transplantation. This was similar in denied and approved cases for all subgroups analyzed (adult, pediatric, cirrhotic, metabolic/structural). This supports the view that denied cases are intrinsically at lower risk.
A higher proportion of approved cases received a transplant compared to patients denied (63.8% vs. 46.6%, P < .0001). Thus, despite approved patients receiving transplants preferentially (as they are supposed to), the increased mortality indicates that they were sicker.
Cox proportional hazard modeling confirmed a trend toward reduced mortality in patients denied accelerated listing compared to those approved, after adjustment for the baseline MPS (P = .055).
The Cox model shows that the difference in mortality is only partly accounted for by differences in baseline MPS. It also shows that referring physicians and RRBs are recognizing factors in the cases receiving approval that are not entirely accounted for by the baseline MPS.
The second important finding of our study was that referring physicians' intuition regarding patient mortality did not contribute any additional information after the baseline (true) MPS was taken into account. In nonsanctioned patients, UNOS does not specify the amount by which the MPS will be increased. The referring physician chooses the score according to the perceived risk of death in the patient.
In cirrhotic patients, Cox proportional hazards modeling showed that only the baseline (laboratory) MPS significantly predicted the risk of death (P = .0003), while the requested MPS (P = .23) had no effect in the model. This is important because it shows that experienced physicians' intuition has poor predictive capacity and adds no additional information to the risk assessment of the true MPS.
This observation held true when only cirrhotic patients were analyzed. This was done because death in all other nonsanctioned categories is not from intrinsic liver disease: patients either become unable to undergo transplantation or have increased mortality posttransplant. Therefore, the predictive value of MPS for death while awaiting transplant was only relevant in cirrhotic patients. Sanctioned cases were excluded because, by definition, prognosis in these conditions is not predicted by the baseline MPS.
This confirms what many studies have shown about the high predictive value for short-term mortality of the MPS,[1][2][5-9] with reported concordance (c)-statistic between 0.83 and 0.92.[1][2][9] More importantly, the absence of increased death in denied cases and the poor ability of referring physicians to predict outcome - but the excellent predictive capacity of the MPS - should reassure UNOS, referring physicians, patients, and the transplant community that patients are being treated fairly. Taken together, these observations show that RRBs function well not only in separating high- from low-risk patients referred to them, but also that the process contributes additional prognostic information, which fine-tunes organ allocation.
The RRB's superior ability compared to that of the referring physician to make the distinction between high- and low-risk cases, probably reflects the increased objectivity of the RRBs, which are removed from the immediate clinical situation. The RRBs also bring a large combined experience because each RRB includes many highly experienced transplant professionals. In addition, referring physicians may make requests based on quality-of-life issues, which would not be reflected in the patient's mortality. UNOS policy specifies transplants will be done first in the sickest patients.
Despite being denied accelerated listing, 45% of the subjects did go on to receive a transplant within the follow-up period of the study. The large number of patients undergoing transplantation despite being denied accelerated listing indicates that the standard UNOS allocation system makes appropriate provision for these patients. An additional safety mechanism for unstable patients denied accelerated listing is the natural increase in MPS. Indeed, Merion et al. have shown that the longitudinal increase in MELD predicts mortality significantly better than the baseline MELD.[10] As UNOS policy evolves, it is likely that patients with increasing MPS will be granted precedence over those with stable MPS.
As expected, our analysis showed that the MPS is not predictive of mortality in patients with UNOS-sanctioned conditions, including cancer, pulmonary, and metabolic and structural liver diseases. The MPS predicts mortality from liver failure, whereas death in nonsanctioned conditions occurs from processes other than complications of liver failure. Indeed, this is the very reason why UNOS sanctions these patients to receive additional MPS points.
Because the increase in MPS was arbitrary in cases referred with nonsanctioned conditions, we evaluated whether the size of the increase (delta-MPS, the difference between the requested MPS and the patient's baseline MPS), affected outcome.
For all nonsanctioned diagnoses, survival was better for delta-MPS greater than or equal to 15 than for delta-MPS less than 15 (P < .00001). The high delta-MPS was associated with improved survival only for cases approved, not for denied cases, indicating the change in score was associated with the improved survival. The finding was true for all subcategories analyzed, including cirrhotic patients only, adult, and pediatric cases.
Cox proportional hazard modeling including delta-MPS into the model with baseline MPS showed that there was only a small effect of delta-MPS but that the predominant effect was due to the baseline MPS (P = .0003).
The baseline MPS in the group with delta-MPS less than 15 (baseline MPS = 18.8) was strikingly higher than those with delta-MPS greater than or equal to 15 (baseline MPS = 12.0; P < .0001). Thus, there was a systematic tendency for referring physicians to request a bigger increase in score for those with low baseline MPS and to request a smaller score for those with high baseline MPS. There was also a significantly shorter time to transplant in cases approved with a high delta-MPS compared to low delta-MPS (P = .0001).
Thus, patients with lower baseline MPS (and hence lower risk) received a greater delta-MPS, resulting in shorter time to transplant. This combination of shorter waiting time in patients at lower risk (as shown by the baseline MPS) accounts for the reduced mortality in this group. It results from the way scores are chosen by the referring physician, as the referring physician chooses the delta-MPS. Transplant physicians therefore need to be aware of the tendency to request a delta-MPS that is inversely proportional to the baseline score. Although it may be appropriate, in the future, to request a delta-MPS that is a factor of the baseline score, further study is required to evaluate this.
Another possible explanation for the discrepancy in mortality associated with the magnitude of delta-MPS is the considerable variability between different organ procurement organizations, within different regions, in the laboratory MPS with which patients undergo transplantation . Organ procurement organizations that perform transplants patients with high MPS may have been requesting smaller increases in their referred patients. We were unable to test this hypothesis. However, we were unable to show a difference in delta-MPS between UNOS regions (P = .36).
Limitations of this study are two-fold. We did not analyze posttransplant survival. This may be important because the delay in getting to transplant in cases denied accelerated listing may affect the posttransplant outcome. This is particularly true of patients referred with conditions other than cirrhosis, but it may also be true in cirrhosis.[8][11]
Second, the numbers of patients in the pediatric, pulmonary, and metabolic/structural groups were small. Therefore, analyses of these groups may have failed to show differences because of insufficient statistical power.
In summary, the RRB process is fair. RRBs do not jeopardize patient survival when they deny requests for accelerated listing. We found RRBs are able to accurately identify cases with a worse mortality risk and approve these for accelerated listing. Despite the approved patients' preferentially receiving transplants, and receiving them earlier, they had higher mortality while awaiting transplantation, confirming the RRBs' risk assessment.
The MELD-PELD score remains by far the most accurate predictor of mortality, but the RRB process does add additional information to the risk assessment, thereby providing an extra safety margin for the sickest cirrhotic patients.
Importantly, we found no evidence that experienced physicians' intuition predicted mortality. This did not contribute anything additional to the predictive value of the MPS.
Finally, referring physicians need to be aware that there is a tendency to request only small increases in MPS in their sickest patients, while requesting larger increases in patients with lower baseline MPS. This may be associated with a worse outcome for the sicker patients receiving the small increases in score.
References
1 Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001; 33: 464-470.
2 McDiarmid SV, Anand R, Lindblad AS. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation 2002; 74: 173-181.
3 Organ distribution: allocation of livers. Policy 3.6. United Network for Organ Sharing Web page. Available at http:// .unos.org/accessed January 14, 2003.
4 Freeman RB, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, et al. The new liver allocation system: Moving toward evidence-based transplantation policy. Liver Transpl 2002; 8: 851-858.
5 Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Testa E, et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: A European study. Gut 2003; 52: 134-139.
6 Brown RS, Kumar KS, Russo MW, Kinkhabwala M, Rudow DL, Harren P, et al. Model for End-Stage Liver Disease and Child-Turcotte-Pugh score as predictors of pretransplantation disease severity, posttransplantation outcome, and resource utilization in United Network for Organ Sharing status 2A patients. Liver Transpl 2002; 8: 278-284.
7 Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, et al. Model for End-Stage Liver Disease (MELD) for predicting mortality in patients with acute variceal bleeding. Hepatology 2002; 35: 1282-1284.
8 Onaca NN, Levy MF, Sanchez EQ, Chinnakotla S, Fasola CG, Thomas MJ, et al. A correlation between the pretransplantation MELD score and mortality in the first two years after liver transplantation. Liver Transpl 2003; 9: 117-123. Links
9 Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for End-Stage Liver Disease (MELD) and allocation of donor livers. Gastroenterology 2003; 124: 91-96.
10 Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl 2003; 9: 12-18. 11 Saab S, Wang V, Ibrahim AB, Durazo F, Han S, Farmer DG, et al. MELD score predicts 1-year patient survival post-orthotopic liver transplantation. Liver Transpl 2003; 9: 473-476.
|
|
| |
| |
|
|
|