| |
Liver transplantation in patients with HIV infection
|
| |
| |
DOWNLOAD A PDF OF THIS ARTICLE
Liver Transplantation
Volume 10, Issue S10, October 2004
Conference: AASLD/ILTS Transplant Course, Boston, MA, 29 October 2004
John Fung 1 *, Bijan Eghtesad 1, Kusum Patel-Tom 1, Michael DeVera 1, Holly Chapman 1, Margaret Ragni 2
1Department of Surgery, Thomas E. Starzl Transplantation Institute, University of Pittsburgh, Pittsburgh, PA
2Department of Medicine, Thomas E. Starzl Transplantation Institute, University of Pittsburgh, Pittsburgh, PA
Key Points
1 Liver transplantation for human immunodeficiency virus (HIV)-positive patients with end-stage liver disease in the era of highly active retroviral therapy has proven to be an effective treatment. The concerns of HIV progression have not been borne out by the growing worldwide experience.
2 CD4 counts are stable and HIV viral load is controllable with medication following liver transplantation.
3 Hepatitis C virus (HCV) coinfection in HIV-positive recipients is universal, but the severity of recurrence does not appear to be different from that in HIV-negative patients with HCV liver disease.
4 Complex pharmacokinetic interactions between the calcineurin inhibitors used for immunosuppression along with protease inhibitors are present, but management directed at recognizing the need for monitoring levels does not appear to increase the risk of toxicity.
5 The degree of immunosuppression from iatrogenic drug therapy and HIV does not lead to increased risk of infectious complications.
Article Text
Abbreviations:
HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; gp, glycoprotein; NRTI, nucleoside transcriptase inhibitors; PI, protease inhibitor; HAART, highly active antiretroviral therapy; OI, opportunistic infection; MMF, mycophenolate mofetil; MPA, mycophenolic acid; GTP, guanosine triphosphate; HCV, hepatitis C virus; HBV, hepatitis B virus; ESLD, end-stage liver disease; AUC, area under the curve.
Human Immunodeficiency Virus (HIV)
Both HIVs, type 1 and type 2, can infect humans and lead to progressive immune deficiency. By far the best studied and the most prevalent strain in the United States is HIV-1, and for the purpose of this overview, the term HIV applies to HIV-1. HIV infection is associated with a spectrum of clinical conditions, from no symptomatology to acquired immunodeficiency syndrome (AIDS; Table 1). AIDS is a syndrome that was first recognized in 1981 and is now defined as the presence of one of more than 20 conditions, including development of one or more opportunistic infections and/or CD4 count less than 200 cells/mm3. In the United States, it is estimated that there are between 750,000 and 1.5 million individuals infected with HIV, with 40,000 new cases added each year.[2] In addition, approximately 40,000 deaths from HIV occur each year, and it is the leading cause of death in men between 25 and 44 years of age. Worldwide, the statistics are even more staggering, with more than 40 million people infected with HIV in 2000. Given the magnitude of this disease, tremendous progress has been made in the understanding of the virus, the process of replication, the mechanisms of inducing immune deficiency, and models for projecting survival. Until the advent of antiviral therapies targeted toward HIV, the natural progression to AIDS could be predicted based on viral load and CD4 counts.
Table 1. Revised 1993 Centers for Disease Control Classification System of HIV Infection
Category A - Asymptomatic HIV infection
- Persistent generalized lymphadenopathy
- Acute retroviral syndrome
Category B - Bacillary angiomatosis
- Oral or recurrent vaginal candidiasis
- Cervical dysplasia
- Prolonged constitutional symptoms
- Oral hairy leukoplakia
- Herpes zoster
- Idiopathic thrombocytopenic purpura
- Listeriosis
- Pelvic inflammatory disease
- Peripheral neuropathy
Category C - CD4 count < 200 cells/mm3
- Cervical cancer
- Chronic herpes simplex
- Coccidioidomycosis
- Cryptococcosis
- Cryptosporidiosis
- Cytomegalovirus
- Histoplasmosis
- HIV encephalopathy/dementia
- Invasive Aspergillus
- Isosporiasis
- Kaposi's sarcoma
- Lymphoma
- Microsporidiosis
- Mycobacterium avium
- Mycobacterium kansasii
- Mycobacterium tuberculosis
- Penicilliosis
- Pneumocystis carinii
- Progressive multifocal leukoencephalopathy
- Pulmonary or esophageal candidiasis
- Recurrent pneumonia
- Salmonellosis
- Toxoplasmosis
Although HIV is a retrovirus belonging to the lentivirus subfamily and is structurally relatively simple, it has a complex replication cycle. The virion is composed of a 2 single-stranded RNA genome packaged together with virally encoded proteins, including reverse transcriptase and structural proteins, as well as elements derived from the host, including cyclophilin A and a membrane composed of elements from both virus and host. The primary target is the CD4-positive T cell, by virtue of high-specificity binding of viral glycoprotein (gp) 120 (gp120), the HIV glycoprotein associated with the trimeric gp41 complex, to the CD4 receptor. Other cells, including monocytes and glial cells, also bear CD4 antigen and can serve as a reservoir for HIV. Fusion of the virus into the target cell also requires the presence of chemokine receptors, such as CXCR4 (lymphotropic strain) or CCR5 (monocytotrophic strain). Other chemokine receptors, such as CCR3, have been shown to function in this manner. Viral fusion is mediated by gp41 and results in deposition of the reverse transcription complex into the cytoplasm. In some HIV strains, cyclophilin A has been implicated as playing an important role in activating reverse transcription. Complementary DNA (cDNA) synthesis is followed by nuclear translocation and nuclear integration facilitated by viral integrase. Viral replication is the culmination of a complex series of steps, including synthesis of viral structural (Gag and Env) and enzymatic (pol) polyprotein precursor and viral RNA synthesis. Viral packaging occurs at the plasma membrane of the infected cells; this results in budding of immature viral particles. Autocatalytic activation of the protease enzyme provides further posttranslational processing of critical polyproteins required for the final maturation and subsequent infectivity. Certain HIV genes, in particular those encoding the envelope proteins and reverse transcriptase, are error prone and explain both the presence of quasispecies and the development of drug-resistant mutations.
The estimated total body pool of HIV is approximately 1010 virions. The kinetics of established HIV infection is notable for significant viral turnover: on the order of 30% of the total body viral load is eliminated on a daily basis and the life cycle in vivo of HIV is estimated at 1.2 days. Associated with this brisk viral turnover is a daily turnover of 5% of the total host CD4+ T-cell pool as part of active HIV replication. This leads to eventual depletion of the CD4+ T-cell pool as the rate of destruction exceeds the ability of the precursor pool to renew the total CD4+ T-cell population. However, a small population of HIV-infected individuals do not follow this timeline of progression to AIDS - 10% to 17% of infected individuals remain clinically free of AIDS 20 years after exposure, and a subset of these patients (less than 5% of all HIV-infected individuals) have stable CD4+ T-cell counts - these patients are termed long-term nonprogressors. Immunological and virological studies of these individuals have failed to demonstrate a consistent pattern of viral and host factors explaining this phenomenon.
Anti-HIV Therapy
Delineation of the critical steps in viral replication and infection has led to development of strategies and treatments designed to reduce viral replication. Specifically, current therapies are directed toward the defined pathways: inhibition of viral binding and/or entry (enfuvirtide - inhibitor of gp41-mediated fusion); inhibiting reverse transcription (nucleoside transcriptase inhibitors [NRTI] and non-nucleoside reverse transcriptase inhibitors [NNRTI]); and inhibiting protease activity (protease inhibitors [PIs]). Since 1997, the vast majority of antiretroviral therapy protocols have included multiple drug therapy. Monotherapy and dual therapies are not recommended in the treatment of HIV-infected patients, primarily due to the high risk of developing resistance. Highly active antiretroviral therapy (HAART) generally consists of 3 active antiviral agents - 2 or more NRTI plus 1 PI or 1 non-nucleoside reverse transcriptase inhibitor, - and has been shown to be associated with significant stabilization of CD4 counts, reduction in the incidence of opportunistic infection (OI), and lower mortality. Clinical studies have demonstrated virological, immunological, and survival benefits associated with the use of HAART regimens.
Other potential approaches to managing HIV infection include novel observations with the use of immunosuppressive agents. For example, it has been reported that HIV replication can be inhibited by cyclosporine and tacrolimus. Both animal and pilot human studies have been conducted, examining the effect of calcineurin inhibitors on HIV infection, presumably by inhibiting T-cell activation and thus cytokine synthesis (which can augment HIV replication), as well as inherent effects mediated by immunophilin receptors. Mycophenolate mofetil (MMF) is another immunosuppressive agent that potentiates the effects of antiviral medications. MMF is a prodrug of mycophenolic acid (MPA), which reversibly inhibits lymphocyte inosine monophosphate dehydrogenase, thereby inhibiting de novo purine nucleotide synthesis and drastically decreasing intracellular guanosine triphosphate (GTP) concentrations both in vitro and in vivo. MPA has been shown to enhance the effectiveness of purine nucleoside analogues.
Liver Disease in HIV-Infected Individuals
The irony of the diminishing mortality associated with HIV infection is the increasingly apparent impact of coinfection with chronic viral hepatitis. HIV-positive patients are at risk for hepatitis C virus (HCV) and hepatitis B virus (HBV) infection and the development of end-stage liver disease (ESLD). The route for HIV acquisition is often the same as the risk for acquisition of viral hepatitis, namely, transfusion of blood products, shared needles, and unprotected sexual practices. The prevalence of HCV coinfection among HIV-positive patients has been reported in the range of 23% to 33%, while the prevalence of chronic HBV coinfection is approximately 9%. In some high-risk HIV-positive groups, such as hemophiliacs receiving lifelong serum-derived factor replacement, the incidence of HCV coinfection has been reported to be as high as 80% in HIV-positive hemophiliacs. It has also been reported that the presence of HIV infection accelerates the progression of chronic liver disease to ESLD. This translates into higher mortality rates from ESLD in HIV-positive patients coinfected with viral hepatitis. In-hospital mortality was 12% in admissions for liver disease in one study, and it was 4.8% in another study. In one study, the reported deaths due to ESLD has grown impressively, from 11.5% in 1991 to 13.9% in 1996, and to 50% in 1998-1999. In HCV- and HIV-coinfected hemophiliac patients, the relative risk of developing ESLD was 3.72, and death secondary to ESLD was 3.81, when compared to a group of non-HIV-infected hemophiliacs with HCV.
In addition to chronic viral hepatitis, other causes of ESLD have been noted in the HIV-positive population. Acute liver failure with lactic acidosis, hepatic steatosis, and mitochondrial DNA injury has been reported as a result of therapy with the D drug reverse transcriptase inhibitors (e.g., zalcitabine, didanosine, and stavudine). These agents are all potent inhibitors of polymerase , compared to other non-D drugs, such as zidovudine, lamivudine, and abacavir. It has also been suggested that the presence of HCV may act in association with the use of D-drug in depleting mitochondrial DNA levels. Other antiviral agents, in particular efavirenz and nevirapine, have also been associated with elevations in liver enzymes and may potentiate the detrimental effects of alcohol. HIV has also been associated with an AIDS-related cholangiopathy, leading to biliary strictures. The causative agent in these cases has been reported to include Cryptosporidium, cytomegalovirus (CMV), and Microspooridia. Lastly, there is some speculation that autoimmunity induced by HIV and/or retroviral antigen mimicry may be involved in primary biliary cirrhosis.
HIV and Organ Transplantation
In spite of the many advances in organ transplantation, the presence of HIV in a patient with end-stage organ failure has been considered a relative or absolute contraindication for transplantation at many centers, for both medical and psychosocial reasons. The conceptual conflict lies in the administration of iatrogenic immunosuppression to the immunocompromised HIV-infected individual, a process that relegates organ transplantation in HIV recipients to the status of a continued enigma. Early reports suggested that the course of HIV infection is accelerated in transplant patients, either due to the effect of immunosuppression or to the role of alloantigenic stimulation of lymphocytes. However, it is difficult to separate the infectious complications associated with HIV from those associated with iatrogenic immunosuppression. A review of the relatively sparse pre-HAART experience concerning the approach and outcome of solid organ transplantation in HIV-positive recipients is limited by the lack of ability to discern HIV-related deaths from normal OI in transplant patients, the lack of staging information (i.e., HIV viral load or CD4+ counts), and the lack of treatment for HIV based on current understanding of antiviral therapy. The use of AIDS OI terminology in the transplant population should be seriously questioned because these infections can also be seen in HIV-negative organ transplant recipients.
The first series of patients who were positive for HIV at the time of transplantation and of patients who acquired HIV (presumably from the donor) were reported by the University of Pittsburgh. In a retrospective serologic survey of organ donors and transplant recipients, 7 HIV-positive recipients had antibodies to HIV-1 prior to liver transplantation, while the other 8 HIV-positive recipients seroconverted after liver transplantation. Of the 15 liver transplant patients, 7 were alive at a mean of 2.75 years. With further follow-up (12.75 years), only 2 liver transplant patients remained alive, both on anti-HIV therapy.
In a series of 4 hemophiliac patients at the University of Pittsburgh, performed from 1982 to 1987 (2 of whom were included in the analysis by Tzakis et al.), 1 patient died in the perioperative period, and 3 survived for varying periods from 4 months to 44 months, before dying from a variety of OI: Pneumocystis, toxoplasmosis, and cryptosporidiosis. These cases preceded the routine use of trimethoprim/sulfamethoxazole and the availability of effective agents against Cryptosporidium. All deaths were classified as AIDS related. In addition, the University of Pittsburgh also reported 2 additional HIV-positive liver transplants under tacrolimus immunosuppression: 1 with chronic HBV and 1 with chronic HCV. The HBV recipient developed HBV recurrence and died at 102 months; the HCV patient suffered from HCV recurrence and survived only 7 months.
Researchers at the University of Minnesota reviewed the overall liver transplantation experience in HIV-infected recipients reported in the literature from 1985 to 1990. Twenty-two cases were identified, 10 HIV positive at the time of liver transplantation and 12 developing HIV perioperatively. They noted that patients infected pretransplant with HIV had a shorter time to progression and a greater risk of dying from AIDS (defined primarily by development of posttransplant OI) or AIDS-related complications than those who acquired HIV in the perioperative period. In additional, the authors noted that in the HIV-positive patients who underwent transplantation and were long-term survivors, good liver function could be demonstrated for an extended period of time. Others have also reported positive outcomes in isolated cases.
Controversies in Liver Transplantation in HIV-Positive Patients
The following concepts summarize the current issues in offering liver transplantation to HIV-positive candidates: (1) a stable HIV-positive candidate will immunologically decompensate with iatrogenic immunosuppression, (2) the viral load will increase and/or the immunosuppression may enhance HIV mutations, (3) the pharmacokinetics and pharmacointeractions of current antiretroviral agents and immunosuppression may lead to subtherapeutic effects or toxicity, (4) recurrent HCV infection along with HIV infection may lead to accelerated fibrosis and graft failure, and (5) the public perception on offering transplantation to HIV-positive recipients will lead to diminished support for donation. Because these concerns have translated into lack of access for HIV-positive patients needing liver transplantation and efforts by third-party payers to deny coverage, the remainder of this article will focus on these issues by examining the current status of liver transplantation in the HAART era.
Liver Transplantation in HIV-Positive ESLD in the HAART Era
As shown in Table 2, there is an accelerating experience in providing liver transplantation for HIV-positive patients in the HAART era. According to the literature, the total number of liver transplants performed in HIV-positive recipients in the HAART era is now 50: the US experience comprises 21 patients, the European experience comprises 27 patients, and the Asian experience comprises 2 patients. HCV was the primary indication, accounting for 68% of causes of liver disease. With varying periods of follow-up, 80% of patients were reported alive.
Table 2. Worldwide Experience with Liver Transplantation in HIV Patients in the HAART Era (see references at end of article)
|
Center
|
Year
|
Reference
|
Number
|
% HCV
|
% Surviving
|
|
King's College, UK
|
1996
|
[58]
|
1
|
100%
|
100%
|
|
Milan
|
1998
|
[59]
|
1
|
0%
|
100%
|
|
Pittsburgh
|
1999
|
[60]
|
1
|
100%
|
100%
|
|
New York
|
1999
|
[61]
|
1
|
100%
|
100%
|
|
Sweden
|
2000
|
[62]
|
1
|
100%
|
100%
|
|
Bonn
|
2000
|
[63]
|
1
|
0%
|
100%
|
|
King's College, UK
|
2001
|
[64][65]
|
5
|
60%
|
40%
|
|
Birmingham, UK
|
2001
|
[66]
|
1
|
100%
|
100%
|
|
Leeds, UK
|
2001
|
[67]
|
1
|
100%
|
0%
|
|
Japan
|
2002
|
[68]
|
1
|
100%
|
100%
|
|
Barcelona
|
2002
|
[69]
|
1
|
NA
|
NA
|
|
Miami
|
2003
|
[70]
|
6
|
50%
|
100%
|
|
Pittsburgh
|
2003
|
[65][70]
|
10
|
80%
|
80%
|
|
UCSF
|
2003
|
[65][71]
|
4
|
25%
|
75%
|
|
Madrid
|
2003
|
[72]
|
1
|
100%
|
100%
|
|
Sweden
|
2003
|
[73]
|
3
|
100%
|
67%
|
|
Taiwan
|
2003
|
[74]
|
1
|
0%
|
100%
|
|
Clichy/Rome
|
2004
|
[75]
|
10
|
70%
|
80%
|
|
Rome
|
2004
|
[76]
|
1
|
100%
|
100%
|
|
Total
|
|
|
51
|
68%
|
80%
|
Abbreviations: NA, not applicable; UCSF, University of California, San Francisco; HIV, human immunodeficiency virus; HCV, hepatitis C virus.
Roland and Stock queried the United Network for Organ Sharing (UNOS) registry for HIV-positive liver transplants performed during the HAART era and found 19 patients.[76] The overall patient survival was 79%, with a median follow-up of 314 days; this was similar to non-HIV-positive liver transplant recipients with a 1-year survival of 88%.
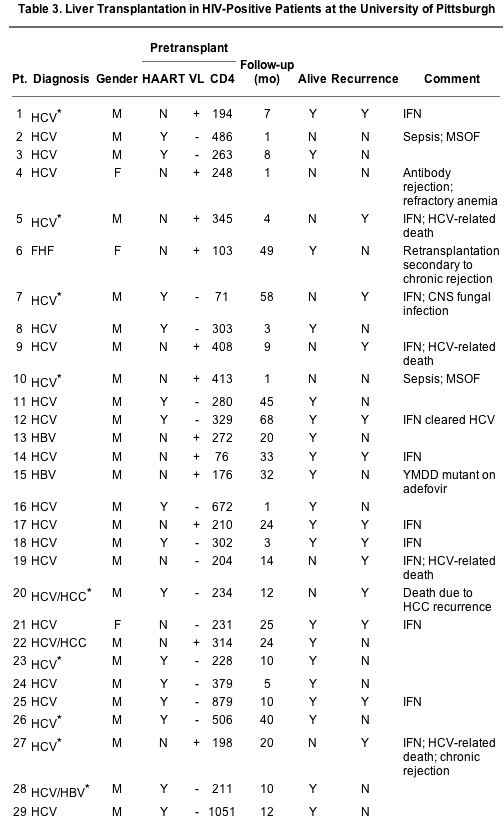
The largest single-center series comes from the University of Pittsburgh. A total of 29 HIV-positive ESLD patients have been recipients of liver allografts alone since 1997 (1 patient received both a liver and kidney). The indications for liver transplantation are shown in Table 3. Overall, 89% were for HCV, 7% were for HBV, and 4% were for fulminant liver failure. Twenty-six percent were hemophiliacs that acquired viral hepatitis from coagulation factor replacement. Two patients also had concomitant hepatocellular carcinoma. The average Model for End-Stage Liver Disease score was 21 (range, 8-46). The vast majority (89%) of patients were male, and the average age at transplantation was 46.6 years (range, 34-66).
|
|
| |
| |
 |
|
| |
| |
Overall, all but 1 patient received some antiretroviral therapy at some point before transplantation; however, only 16 (55%) of recipients were taking HAART at the time of liver transplant. Twelve patients had been discontinued from HAART for varying periods before transplantation, resulting in 12 patients who had HIV viral loads in the detectable range. In patients with liver failure, there can be significant difficulties in dosing HAART, given the added hepatoxicity of NRTI and the altered pharmacokinetics of PI metabolism. We have taken the position of allowing ESLD HIV-positive patients to have detectable HIV loads prior to transplantation, as long as the pattern of drug history and demonstrated HAART resistance pattern can predict suppression of HIV replication following reinstitution of HAART once normal liver function returns after liver transplantation.
Nine patients died during the follow-up period, which averaged 18 months (range, 1-68). Perioperative complications not related to HIV (accelerated humoral rejection with a strongly positive crossmatch and sepsis) contributed to 3 deaths within 30 days. One late death was related directly to an infectious cause (a central nervous system fungal infection at 58 months after liver transplantation). One patient succumbed to recurrent hepatocellular carcinoma. Recurrent HCV was the principal cause of or was a contributing factor in 4 deaths (in one, treatment for acute and chronic rejection exacerbated HCV, and both complications were equal culprits in worsening liver failure). Although the 1-year survival of all patients was 76% and the overall survival was 69%, of those that survived more than 30 days posttransplant, the 1-year patient survival was 89% and the overall survival was 77%. In all cases, liver transplantation reversed the stigmata of acute and chronic liver failure, including ascites, encephalopathy, muscle wasting, fatigue, hypersplenism, and jaundice.
Due to the skewed nature of liver disease in the HIV population - i.e., higher incidence of viral hepatitis - the survival outcomes would be more appropriately compared to a group of viral hepatitis, non-HIV-positive liver transplant recipients. HIV-positive patients with HBV infection, for which effective prophylaxis against recurrence exists using hepatitis B immune globulin (HBIG) and lamivudine, do not appear to suffer recurrence and have excellent short- and long-term survival rates. However, analysis of the subset of HCV- and HIV-coinfected patients points to a potential concern of HCV recurrence on long-term survival. Although it is important to note the poor outcomes in HCV- and HIV-coinfected patients from the King's College group (in which recurrence led to decreased survival), it should be noted that their experience was in the very early period, before the complexity of HAART and immunosuppression was appreciated. The combined experience of the Universities of Miami and Pittsburgh points to a more optimistic outcome, with a 1-year survival rate no different from that for HCV-positive, HIV-negative patients. At the University of Pittsburgh, HCV recurrence was treated with ribavirin and interferon therapy; this was done in 12 HCV patients (52% of HCV patients surviving more than 1 month), although only 2 patients achieved sustained HCV clearance. The impact of HCV recurrence and disease progression in the HIV-positive liver transplant recipients does not allow us to draw a definitive conclusion about the potential for poorer outcomes. However, the issue of HCV recurrence in liver transplantation is certainly of concern in general, in light of recent data showing worsened long-term survival in the whole population of HCV-positive patients. Certainly, approaches and new agents to combat recurrent HCV will benefit all HCV patients, regardless of their HIV status.
Choice of Immunosuppression
Given the ever-growing number of immunosuppressive agents for the prevention and treatment of rejection in clinical transplantation, there are numerous approaches to managing HIV-positive patients receiving liver transplantation. Calcineurin inhibitors, cyclosporine, and tacrolimus remain the mainstays of baseline immunosuppression following liver transplantation and are critical in minimizing the incidence of rejection. Corticosteroids are also utilized in most regimens; however, the use of other adjunctive agents, such as azathioprine, MMF, and rapamycin, has not been uniformly incorporated into immunosuppressive protocols in HIV-positive recipients. Antilymphocyte antibodies, ranging from panlymphocyte antibodies - e.g., antithymocyte globulin (Thymoglobulin, ATGAM) and alemtuzumab (Campath 1H) - to anti-T-cell antibodies (e.g., OKT3) to anti-interleukin 2-receptor antibodies - e.g., basiliximab (Simulect) and dacluzimab (Zenapax) - also have not found a place in immunosuppressive protocols for HIV positive patients, the rationale being based on the poorer early experiences with OKT3 in HIV-positive patients.
In any case, the paramount goal in the selection of a given immunosuppressive regimen is to minimize rejection, while preventing posttransplant OI. Recently, there have been intriguing reports on the potential effect of immunosuppressive agents in the treatment of HIV patients. HIV utilizes cellular cyclophilin for structural protein processing (important in the assembly of HIV virions) as well as for subsequent infectivity. Cyclosporine interferes with cyclophilin activity and inhibits HIV replication in vitro through inhibition of lymphocyte activation and specific inhibition of cyclophilin-HIV gag polyprotein interaction. However, in a randomized, double-blind, placebo-controlled trial, low-dose cyclosporine (2 mg/kg) did not affect HIV viral load or CD4+ T-cell counts.[83] Nevertheless, the results of an earlier study showed that liver transplant patients acquiring HIV in the perioperative period from contaminated blood or allografts under cyclosporine immunosuppression appeared to experience a cumulative lower risk of HIV progression than those that were already infected.
The possibility that corticosteroids, through inhibition of lymphocyte activation and thus cytokine production, might also positively influence the impact of HIV has also been examined. A randomized, double-blind, placebo-controlled trial examining a short course of prednisone (0.5 mg/kg/d for 8 weeks) in patients with CD4 counts averaging 131 cells/L showed that this treatment was well tolerated and reasonably safe in patients with stable HIV disease, although no major HIV benefit was observed. On the other hand, a similar study in patients with baseline CD4 counts greater than 200 cells/L noted an increase (>40%) in CD4+ T cells, although side effects were more notable.
Targeting reverse transcriptase with nucleoside analogues provides another potential area for immunosuppressive drug/HAART interactions. MPA, through its inhibition of GTP synthesis, acts synergistically with guanosine analogues such as abacavir in the inhibition of HIV replication. However, pilot clinical trials that attempted to exploit this robust in vitro phenomenon for salvage in HAART-resistant HIV-positive patients did not demonstrate a sustained benefit, although transient drops in HIV viral load were obtained. Nevertheless, these studies demonstrated that MMF could be safely administered and provide reassurance that it can be used safely as part of a maintenance immunosuppressive regimen.
Although modulation of host immunity with immunosuppressive agents has yet to demonstrate a clear clinical benefit to HIV-infected patients, available data indicate that these maintenance immunosuppressive agents can be used in the context of liver transplantation for HIV-positive patients without dire consequences for safety.
Rejection
One area in which there is little scientific knowledge is the impact of HIV on the risk of rejection and vice versa. HIV-positive patients have variable levels of immune deficiency, and some investigators have suggested that there are diminished rates of rejection in HIV positive patients and thus they may be able to reduce immunosuppression without precipitating rejection. This finding has not been borne out in the early posttransplant period, and acceptance of this approach may be quite harmful. Although HIV-positive patients may be able to reduce the level of immunosuppression after transplantation, this is a common phenomenon in non-HIV recipients. In fact, it is not surprising that others have shown rates of rejection equal to or even greater than non-HIV-positive transplant candidates. This is due to the fact that an estimated 10% of all CD4+ T cells can respond to allogeneic stimuli; thus, total CD4+ T-cell counts must be markedly suppressed before the number of alloreactive recipient T cells would obviate the development of rejection. In addition, HIV-infected patients have been shown to have dysregulated B-cell function and polyclonal gammopathy. This has been postulated to explain the unexpectedly high rates of rejection seen in a recent kidney transplant study.
Early reports using cyclosporine-based immunosuppression were associated with high rates of rejection and the need for antilymphocyte antibody therapy, which was also associated with high risk of OI and death.[48] Whether this increased morbidity and mortality is due to increased risk of OI, or to the potential impact of allogeneic stimulation of HIV-infected CD4+ T cells on increasing HIV replication and thus progression of HIV replication, has not been determined. While the optimal approach to immunosuppression in HIV-positive recipients has not been determined, it appears reasonable to approach these patients with the same intensity of early immunosuppression as in non-HIV-positive patients. Nevertheless, this area will require further study.
Choice of HAART Regimen
In general, the principles for HIV therapy in transplant patients should be the same as for nontransplant patients (Table 4). There are growing numbers of agents and novel compounds to inhibit replication of HIV that have been introduced into the clinical arena. Today, most treatment regimens include at least 2, and sometimes 3 different classes of antiretroviral therapy. The HIV clinician is faced with changing recommendations, some based on efficacy, others based on resistance patterns, and still others based on side effects of antiviral agents, either alone or in combination. These recommendations are then overlaid on the patient's individual characteristics, necessitating individualized assessment and monitoring of response to HAART. The necessity of continuous HAART after liver transplantation has not yet been demonstrated, although it has been suggested that patients who cannot tolerate HAART posttransplant do poorly. Whether this is due to the lack of HAART or the inability to tolerate HAART due to recurrent HCV has not been determined. There is also uncertainty about the need for HAART in long-term nonprogressing HIV-positive patients.
|
|
| |
| |
 |
|
| |
| |
Pharmacointeractions
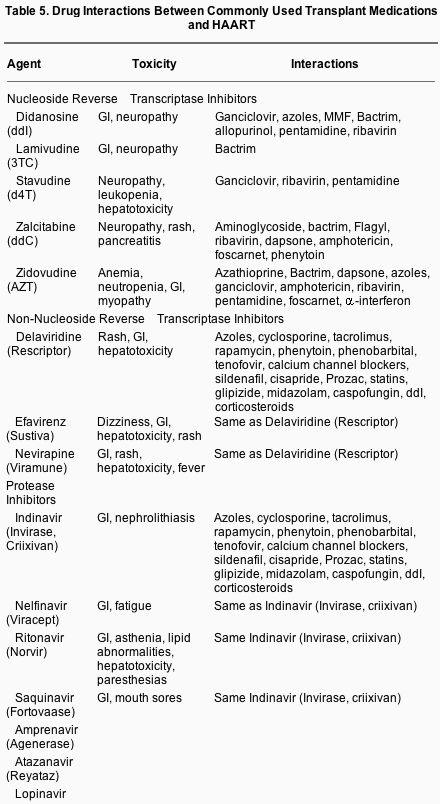
Cyclosporine (CSA), tacrolimus, and rapamycin are primarily metabolized by cytochrome P450 3A2 and 3A4 isoenyzmes and are subject to pharmacokinetic interactions by other drugs that induce or inhibit this enzyme activity. These agents are also substrates of the p-glycoprotein system, also known as the multiple drug resistance transport system. In addition, the PI agents are similarly metabolized by this group of enzymes, some acting as inducers and others as inhibitors of these metabolic enzyme pathways (Table 5; see also http://www.hiv-druginteractions.org and http://www.proojinf.org for additional drug interactions). Drug interactions in hepatic metabolism between calcineurin inhibitors and PIs have been well described in liver transplant recipients. Several studies have indicated significant pharmacological interactions between tacrolimus and PIs, including nelfinavir, ritonavir, and lopinavir. After liver transplantation and before reinstitution of HIV therapy, tacrolimus levels were adjusted to maintain therapeutic levels. Following resumption of PIs, tacrolimus levels rose dramatically, resulting in toxicity. Tacrolimus dosing was markedly reduced to minimize levels of tacrolimus and resultant toxicity. Such interactions are frequent with combined tacrolimus and PI use, and the average maintenance dose of tacrolimus in the University of Pittsburgh experience is 1 mg/wk.
|
|
| |
| |
 |
|
| |
| |
Similar findings have been noted with cyclosporine and PI interactions. Brinkman et al. described a patient receiving simultaneous saquinavir and cyclosporine, in whom the addition of nelfinavir elevated cyclosporine area-under-the curve (AUC) by 5- to 10-fold. With a 50% reduction in the CSA dose, in the setting of continued saquinavir use the CSA AUC was still 90% of that without the use of PIs. Like tacrolimus, when used in combination with nelfinavir, saquinavir, or ritonavir, cyclosporine levels may need to be dosed less frequently than once a day.
Although not a calcineurin inhibitor, rapamycin belongs to the class of macrolide antibiotics and is metabolized by the same cytochrome P450 pathway. Jain et al. reported significant drug interactions in a liver transplant patient on sirolimus maintenance immunosuppression to which nelfinavir was added.
Therapeutic monitoring of cyclosporine, tacrolimus, or rapamycin levels is critically important, not only when PI use is instituted but also when it is stopped. In one case, the local physician treating HIV elected to take the patient off of HAART therapy (drug-free holiday). The elimination of the PI caused drastic reduction in tacrolimus levels, precipitating acute rejection that evolved into chronic rejection.[95] This case also highlights the critical need for those managing transplant immunosuppression and those managing HIV medications to communicate before making adjustments in either medication. Unfortunately, the drug interactions are sufficiently complex that drug dosing must be determined empirically for each patient. Although HIV PI levels are not routinely measured, there is reason to consider studying the utility of monitoring of PI levels in patients receiving tacrolimus, cyclosporine, or rapamycin. In one report in which saquinavir levels were measured, the AUC was 4 to 11 times higher than in patients receiving saquinavir but not cyclosporine.
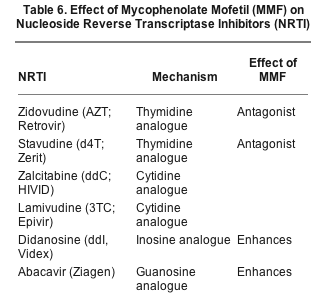
Drug interactions between anti-HIV and antirejection and transplant medications are not only limited to calcineurin inhibitor and PI use. As noted earlier, MPA is a potent, selective, noncompetitive, and reversible inhibitor of inosine monophosphate dehydrogenase, an enzyme involved in the synthesis of deoxyguanosine triphosphate (dGTP). Reduction in intracellular dGTP concentrations induced by MPA is expected to increase the antiretroviral activity of abacavir, a guanosine analogue. In vitro studies show that MPA synergistically increases the antiviral effect of abacavir, didanosine, and tenofovir against multi-NRTI-resistant HIV strains. Antagonism due to inhibition of thymidine kinase has been noted with MPA (and MMF) plus the thymidine analogues zidovudine and stavudine (Table 6). This also raises the important concern of whether the mitochondrial toxicity of NRTI will be potentially augmented by the effect of MPA. Mitochondrial toxicity and lactic acidosis has been linked to the use of D drugs such as didanosine, stavudine, and zalcitabine and is attributed to the damage of mitochondrial polymerase by these agents. Patients with this syndrome can present with lethargy, malaise, hepatitis, peripheral neuropathy, pancreatitis, and other end-organ damage. Failure to identify this syndrome may result in death from hypoxia and lactic acidosis. This has also been suggested to be a potential area for enhanced toxicity.
|
|
| |
| |
 |
|
| |
| |
Ribavirin used in combination for HCV therapy has been shown to inhibit phosphorylation of zidovudine, stavudine, and zalcitabine. Ribavirin may also increase the potency of didanosine and improve anitviral activity. Since ribavirin is also a known inhibitor of inosine monophosphate dehydrogenase, this would add another level of potential interaction with MPA and NRTI and could account for enhanced toxicity. In a report of an HCV-coinfected HIV patient on HAART, MMF, and ribavirin, with lactic acidosis and a liver biopsy revealing 80% microsteatosis, electron microscopy revealed abnormalities in mitochondrial structure consistent with NRTI toxicity.
Opportunistic Infections
OI refers to the development of infectious disease in individuals with significant defects in host defenses. The pathogens responsible for this infection often lack intrinsic virulence and therefore require an immune or inflammatory defect to establish infection.
Probably the single most important achievement in the management of the immunocompromised patient are the improvements in the strategies for detection, prevention, and treatment of OI. For prophylaxis, the routine addition of trimethoprim/sulfamethoxazole has reduced the incidence of Pneumocystis jiroveci (formerly Pneumocystis carinii), Toxoplasmosis gondii, and Listeria monocytogenes. The development of assays to detect OI when the infection is subclinical or at the earliest onset of disease allows for targeted preemptive therapy. Such is the case in CMV, in which pp65 antigenemia or CMV polymerase chain reaction monitoring allows for earlier detection and better assessment of efficacy of treatment.[99] However, the utility of early detection would not be of benefit if effective agents were not available for treatment. The development of ganciclovir, first intravenously and now orally, has reduced the morbidity and mortality of CMV infection.[100] While similar assays are being developed for fungal infections and Epstein-Barr viral infections, these tests remain in the developmental phase.
The risk of OI in immunosuppressed HIV-positive transplant patients does not appear to be above and beyond the risk of immunosuppressed HIV-negative transplant recipients using current-day prophylaxis, monitoring, and treatment. The ability to attain routine suppression of HIV viral loads on HAART is associated with stabilization or improvement in CD4 counts, which has been shown to decrease OI in HIV-positive patients.
Current Recommendations for Liver Transplantation in HIV-Positive
HIV-infected ESLD patients should be considered for liver transplantation if they meet standard medical criteria for inclusion/exclusion as currently practiced by a multidisciplinary transplant team and meet UNOS criteria for listing. Added institutional requirements include expertise in HIV management, a transplant pharmacologist with expertise in drug interactions, a transplant infectious diseases specialist, and a fiscal administrator knowledgeable in the area of insurance coverage. In addition to the standard transplant evaluation process, CD4+ T-cell counts, quantitative HIV loads, and current/prior responsiveness to antiviral therapy must be obtained. Patients who demonstrate drug resistance can be considered if an effective antiretroviral drug regimen can be devised; drug-resistance pattern determinations may be required if a clear history of antiviral therapy cannot be obtained in the presence of detectable HIV viral load. Clinical responsiveness to antiretroviral therapy is defined as suppression of HIV RNA to less than 0.4 × 103 copies/mL (using the Chiron assay). Individuals with severe liver dysfunction preventing tolerance of antiretroviral therapy should be considered if they have shown prior responsiveness to, or are naive to HAART. It has been suggested that HAART be stopped before transplantation in order to allow wild type virus [to] rebound; however, this approach has not been proven. These same authors have suggested that lamivudine-resistant hepatitis B may pose a problem in patients with HIV and HBV. However, the options of using high-dose hepatitis B immune globulin and/or the promising experience with adefovir and tenofovir disoproxil for lamivudine-resistant HBV appear to effectively suppress HBV recurrence.
Exclusion criteria are continuing to evolve as more experience is gleaned. Those conditions known to negatively affect survival after liver transplantation, such as renal failure, requirement for life support, and advanced malnutrition are risk factors that have excluded patients from active consideration. The criteria for OI exclusions include a previous OI within the previous 6 to12 months; previous Kaposi's sarcoma (given the high rate of recurrence); and patients with evidence of JC polyoma viral infection. In the 29 cases of liver transplant at the University of Pittsburgh, 6 patients had a remote history of Pneumocystis carinii pneumonia with posttransplant prophylaxis, and none of them experienced PCP recurrence. While most centers that are performing kidney transplantation in HIV-positive candidates have used a CD4 cutoff of 200 cells/mm3 as being the lower threshold for consideration, liver transplant patients have hypersplenism that may render the CD4 count artifactually low and may not actually reflect the overall status of the patient. Thus, lower CD4 counts for these patients can be accepted based on a relative comparison of the CD4 count with the absolute neutrophil count. Lastly, given the complexity of posttransplant monitoring and complex drug regimens, a strong history of noncompliance should be considered a contraindication; however, it should be recognized that due consideration should be given on an individual basis to extenuating circumstances, e.g., long-term nonprogressors.
Because of a relatively high rate of acute cellular rejection in HIV-positive patients, calcineurin-based immunosuppression should be used, with or without steroids and with or without other adjunctive agents, such as MMF. Although we have avoided using antilymphocyte induction, use of induction antibodies has been used in HIV-positive kidney transplant recipients without any apparent detrimental effects on HIV or increased risk of OI (Dr. Henke Tan, University of Pittsburgh, personal communication, July 2004).
With respect to the selection of HAART, very often the choice of antiretroviral medications is dictated by the past history of their use and the patient's responsiveness to various regimens. In our liver transplant patients, HAART has utilized PI combination antiretroviral therapy before (unless not tolerated because of severe liver dysfunction) and after transplantation, when the total bilirubin has fallen to 2 mg/dL. Care should be taken in prescribing antiretroviral medications to continue already successful combinations used by the patient and to avoid drugs that are associated with clinical resistance in the patient. It is critical to modify doses of cyclosporine, tacrolimus, and rapamycin - based on pharmacokinetic interactions with HAART that includes PI) - based on their respective blood levels routinely monitored after transplantation. In addition, HAART will pose a challenge in patients with HCV due to the augmented risk of hepatotoxicity.
Because HCV is the most common coinfection in the HIV-positive patient with ESLD, the issues of HCV recurrence that plague non-HIV-positive liver transplant recipients also apply to the HIV-positive patient. Although the guidelines for treatment of HCV recurrence are evolving, HCV treatment should be initiated posttransplant when there is liver biopsy documentation of recurrent HCV infection and disease is moderate -to severe or progressive. A liver biopsy should be obtained prior to treatment and a HAI score greater than 8 and/or fibrosis stage greater than 2 should be considered as the threshold for treatment with interferon and ribavirin. However, these agents are not without substantial side-effects: ribavirin is excreted by the kidney, and given the high incidence of renal dysfunction in liver transplant patients, dosing should be started at low levels and increased as tolerated. The contraindications for interferon and ribivarin use are shown in Table 7.
|
|
| |
| |
 |
|
| |
| |
In addition, the added risk to medical and surgical teams of HIV exposure is not irrelevant - this argument has been put forward as a reason not to perform transplants at all in this population. All members of the surgical team should be made aware of risks and a treatment plan for prophylaxis should be determined before initiating the transplant procedure.
Future Directions
A multicenter prospective study funded by the National Institutes of Health and coordinated by the University of California, San Francisco, was designed to evaluate various outstanding issues in the use of liver and kidney transplantation in people with HIV disease. The long-range goals are (1) to provide patients and clinicians with information regarding the HIV-specific risks of transplantation, (2) to provide clinicians with information necessary to manage immunosuppressive and HAART medications together, and (3) to understand underlying basic science mechanisms that explain patient outcomes so that clinical management may be adjusted to maximize these outcomes. The study anticipates enrolling 150 kidney transplant recipients and 125 liver transplant recipients over 3 years with 2 to 5 years of follow-up. The primary endpoints are subject survival and graft survival; secondary endpoints include (1) opportunistic complications and changes in CD4+ T-cell counts and HIV-1 RNA levels; (2) viral markers and host responses to viral copathogens, including hepatitis B and C and herpesviruses; (3) rejection rates and markers of alloresponse; and (4) pharmacokinetic interactions between immunosuppressive agents and the hepatically metabolized antiretroviral agents.
Conclusions
We believe that while there should be further accrual and follow-up of these patients to assess long-term benefits and risks, there is ample evidence to support the application of this life-saving procedure to selected HIV-positive patients with ESLD. The growing experience summarized here suggests that liver transplantation is effective in selected HIV-positive patients.
There have been significant advances in the management of transplant patients and in the management of HIV infection that have allowed liver and other transplant procedures to be successful. The successful application of other transplant procedures, such as kidney and heart, highlights the growing acceptance of transplantation in HIV-positive patients. Indeed, a recent editorial supports broader application of all solid-organ transplant procedures for HIV-positive patients.
REFERENCES
58 McCarthy M, Gane E, Pereira S, Tibbs CJ, Heaton N, Rela M, et al. Liver transplantation for haemophiliacs with hepatitis C cirrhosis. Gut 1996; 39: 870-875. Links
59 Caccamo L, Colledan M, Rossi G, Gridelli B, Maggi U, Vannelli A, et al. Post-transplant primary disease does not influence 6-year survival after liver transplantation beyond 1 year. Transpl Int 1998; 11( suppl 1): S212-S220. Links
60 Ragni MV, Dodson SF, Hunt SC, Bontempo FA, Fung JJ. Liver transplantation in a hemophilia patient with acquired immunodeficiency syndrome. Blood 1999; 93: 1113-1114. Links
61 Sheikh AM, Wolf DC, Lebovics E, Goldberg R, Horowitz HW. Concomitant human immunodeficiency virus protease inhibitor therapy markedly reduces tacrolimus metabolism and increases blood levels. Transplantation 1999; 68: 307-309. Links
62 Schvarcz R, Rudbeck G, Soderdahl G, Stahle L. Interaction between nelfinavir and tacrolimus after orthotopic liver transplantation in a patient coinfected with HIV and Hepatiitis C virus (HCV). Transplantation 2000; 69: 2194-2195. Links
63 Schliefer K, Paar WD, Aydemir G, Wolff M, Rockstroh JK, Spengler U, Sauderbruch T. Orthotopic liver transplantation in a 33-year-old patient with fulminant hepatitis B and HIV infection. Dtsch Med Wochenschr 2000; 125: 523-526. Links
64 Prachalias A, Anton P, Taylor C, Srinivasan P, Muiesan P, Wendon J, et al. Liver transplantation in adults coinfected with HIV. Transplantation 2001; 72: 1684-1688. Links
65 Ragni M, Belle SH, Im KA, Neff G, Roland M, Stock P, et al. Survival of human immunodeficiency virus-infected liver transplant recipients. J Infect Dis 2003; 188: 1412-1420. Links
66 Gow PJ, Mutimer D. Liver transplantation for an HIV-positive patient in the era of highly active antiretroviral therapy. AIDS 2001; 15: 291-292. Links
67 Tolan DJ, Davies MH, Millson CE. Fibrosing cholestatic hepatitis after liver transplantation in a patient with Hepatitis C and HIV infection [letter]. N Engl J Med 2001; 345: 1781. Links
68 Sugawara Y, Ohkubo T, Makuuchi M, Kimura S, Morisawa Y, Tachikawa N, Oka S. Living donor liver transplantation in an HIV-positive patient with hemophilia. Transplantation 2002; 74: 1655-1656. Links
69 Rafecas A, Rufi G, Fabregat J, Xiol X. Liver transplantation in a patient infected by human immunodeficiency virus. Med Clin (Barc) 2002; 119: 596. Links
70 Neff GW, Bonham A, Tzakis AG, Ragni M, Jayaweera D, Schiff ER, et al. Orthotopic liver transplantation in patients with human immunodeficiency virus and end-stage liver disease. Liver Transpl 2003; 9: 239-247. Links
71 Stock PG, Roland ME, Carlson L, Freise CE, Roberts JP, Hirose R, et al. Kidney and liver transplantation in human immunodeficiency virus-infected patients: A pilot safety and efficacy study. Transplantation 2003; 76: 370-375. Links
72 Gonzalez Alonso R, Barcena R, Blesa C, Garcia M, Moreno A, Fortun J, et al. Liver transplantation in a patient coinfected with human immunodeficiency virus and hepatitis C virus. Transplant Proc 2003; 35: 1846-1847. Links
73 Nowak P, Schvarcz R, Ericzon BG, Flamhole L, Sonnerborg A. Follow-up of a antiretroviral treatment in liver transplant recipients with primary and chronic HIV type 1 infection. AIDS Res Hum Retroviruses 2003; 19: 13-19. Links
74 Jeng LB, Lee WC, Hung CM, Yu MC, Kuo LM, Chen MF. Liver transplantation in a patient with human immunodeficiency virus infection: A case report. Transplant Proc 2003; 35: 361. Links
75 Boschetto A, Ettorre GM, Durand F, Dondero F, Francoz C, Soommacale D, et al. Is anti-retroviral treatment after transplantation in HIV positive patients always necessary? [abstract no. 14]. 10th Congress of the International Liver Transplantation Society, Kyoto, Japan, 10 June 2004. Liver Transpl 2004; 10: C6. Links
76 Roland M, Stock PG. Review of solid-organ transplantation in HIV-infected patients. Transplantation 2003; 75: 425-429. Links
|
|
| |
| |
|
|
|