 |
 |
 |
| |
96-Week Results from '045' Study Comparing Reyataz/r to Kaletra
|
| |
| |
Reported by Jules Levin
The final oral presentation at the end of today's final conference day was "Long-term Efficacy and Durability of Atazanavir With Ritonavir or Saquinavir vs Lopinavir/Ritonavir in HIV-Infected Patients With Multiple Virologic Failures" reported by Margaret Johnson (Royal Free Hospital, London, UK). The primary analysis for this study was at week 48 and this has been previously reported.
This study is a randomized, open-label trial in treatment-experienced patients who failed 2 or more ART regimens containing 1 or more PI, NNRTI, and NRTIs. Patients were randomized equally at baseline to 1 of 3 regimens: atazanavir (ATV) 300 mg once daily plus ritonavir (RTV) 100 mg once daily, or Kaletra (lopinavir 400 mg twice daily plus 100 mg ritonavir twice daily), or ATV 400 mg once daily plus saquinavir 1200 mg once daily. There were about 120 patients randomized to each of these 3 study arms. For the first two weeks of the study patienys were maintained on the NRTIs they were taking. Then they switched to tenofovir plus a second NRTI. The second NRTI was selected by genotypic testing. The recommended dose for ddI was reduced to 250 mg, depending on weight, due to the interaction with tenofovir (TDF).The ITT analysis Treatment Response Without Prior Failure (TRWPF). TRWPF used a response definition of a minimum of two sequential HIV RNA measurements
As you may know the FDA issued a warning supported by results from a few studies regarding use of ddI+tenofovir along with a NNRTI in patients with high viral load (>100,000 copies/ml). In study 045 patients initially received tenofovir plus a second NRTI selected by genotypic testing at the beginning of the study. There was a higher than expected early viral failure rate in patients with a viral load >100,000 copies/ml who took an NNRTI plus ddi+tenofovir. BMS provided an analysis of patients in study 045 who received Kaletra or ATV/r along with ddI+tenofovir. They did not report response by viral load over or below 100,000 copies/ml, but there was no evidence of a problematic failure rates. Here is the data they reported:
WEEK 48, % <50 copies/ml
(51% of study patients were treated with ddI; p-values related to data below reported as non-significant)
All ATV/r regimens: 38%
ATV/r -- ddI regimens: 36%
ATV/r -- non-ddI regimens: 39%
All LPV/r regimens: 46%
LPV/r -- ddI regimens: 50%
LPV/r -- non-ddI regimens: 43%
Week 96, % <50 c/ml
All ATV/r regimens: 30%
ATV/r -- ddI regimens: 32%
ATV/r -- non-ddI regimens: 31%
All LPV/r regimens: 33%
LPV/r -- ddI regimens: 36%
LPV/r -- non-ddI regimens: 31%
045 STUDY RESULTS
BASELINE PATIENT CHARACTERISTICS
The patient characteristics were similar in each of the treatment groups:
Age: 40 yrs; 21$ females; 60% white, 22% lation, 16% black; 28% had AIDS; 17% had HCV or HBV; median HIV RNA: 4.44 log copies/ml; HIV RNA >100,000 copies/ml: 25%; median CD4 count: 283-317; CD4 <200: 30%.
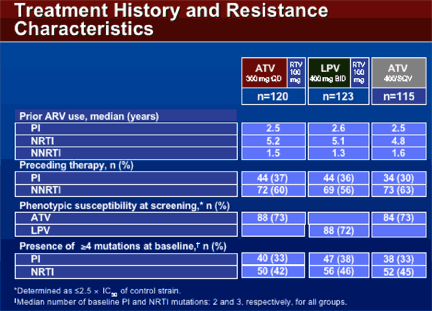
TREATMENT HISTORY & RESISTANCE CHARACTERISTICS
The median number of baseline PI & NRTI mutations were 2 and 3, respectively, for all groups. The median years of prior ARV use was similar between the groups: 2.5 yrs of PI use, 5 yrs of NRTI use, 1.4 yrs of NNRTI use. 36% of patients were on a PI and 56-60% were taking an NNRTI immediately before entering the study. 73% of the patients randomized to ATV/r were phenotypically susceptible (<2.5xIC50) to ATV & 72% of patients randomized to LPV/r were phenotypically susceptible. 33% of the patients in the ATV/r arm had 4 or more PI mutations & 38% in the LPV/r arm had 4 or more PI mutations. 42% in the ATV/r arm & 46% in the LPV/r arm had 4 or more NRTI mutations.
|
|
| |
| |
 |
|
| |
| |
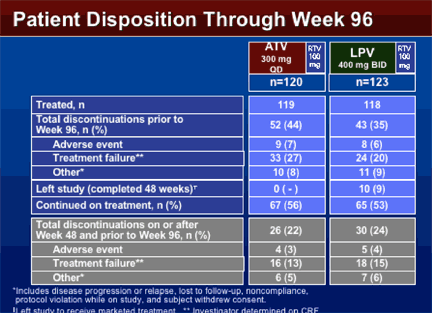
PATIENT DISPOSITION THROUGH WEEK 96
TOTAL DISCONTINUATIONS
44% taking ATV/r & 35% taking LPV/r discontinued prior to week 96--
--due to adverse events: 7% in ATV.r, 6% in LPV/r;
--due to treatment failure*: 27% in ATV/r, 20% in LPV/r
--other**: 8% ATV/r, 9% LPV/r
left study (completed 48 weeks)***: 0% in ATV/r, 9% (n=10) in LPV/r
continued on treatment: 56% taking ATV/r, 53% taking LPV/r
TOTAL DISCONTINUATIONS ON OR AFTER WEEK 48 & PRIOR TO WEEK 96:
22% taking ATV/r, 24% LPV/r
--Due to AE: 3% ATV/r, 4% LPV/r
--Treatment failure*: 13% ATV/r, 15% LPV/r
--Other**: 5% ATV/r, 6% LPV/r
*investigator determined on CRF (I think this includes viral or clinical)
**includes disease progression or relapse, lost to follow-up, non-compliance
***left study to receive marketed product: 10 patients taking LPV/r stopped & left study to take other ART; 5 had detectable VL & 5 were undetectable
|
|
| |
| |
 |
|
| |
| |
PRIMARY ENDPOINT: Time-averaged Difference in HIV RNA Levels Through 96 weeks
ATV/r: -2.29 log copies/ml (n=64)
LPV/r: -2.08 log copies/ml (n=65)
[ATV/r-LPV/r TAD CI: +0.14 (-0.13, 0.41)]
VIROLOGIC RESPONSE: <400 copies/ml through 96 weeks (ITT, TRWPF)
ATV/r: 42%
LPV/r: 43%
[Difference Estimate: ATV 300/r-LPV/r: -1.4 995% CI: -13.9, 11.0)]
VIROLOGIC RESPONSE: <50 copies/ml through 96 weeks (ITT, TRWPF)
ATV/r: 30%
LPV/r: 33%
[Difference Estimate: ATV 300/r-LPV/r: -3.3 (95% CI: -15.0, 8.4)]
VIROLOGIC RESPONSE: <400 c/ml through 96 weeks, On Treatment
ATV/r: 84% (n=67)
LPV/r: 82% (n=65)
(Difference Estimate: ATV 300/r: 2.0 [95% CI: -10.9, 15.0])
VIROLOGIC RESPONSE: <50 c/ml through 96 weeks, On Treatment
ATV/r: 72% (n=67)
LPV/r: 72% (n=65)
[Difference Estimate: ATV 300/r-LPV/r: -0.7 (95% CI: -16.0, 14.7)]
MEDIAN CHANGE in CD4 COUNT through 96 weeks
ATV/r: +160
LPV/r: +142
SAFETY
Grade 2-4 Related Adverse Events Through 96 Weeks
| ATV/r | LPV/r | | AEs leading to D/C, N(%) | 10 (8) | 9 (8) | | Grade 2-4 Related AEs >2%, % | | GI disorders | 9 | 19 | | Diarrhea | 3 | 13 | | Nausea | 3 | 2 | | Jaundice* | 7 | 0 | | Scleral icterus | 3 | 0 | | Myalgia | 4 | 0 | | Lipodystrophy | 3 | 3 |
*no discontinuations due to jaundice or scleral icterus, 6% jaundice at week 48in LPV/r there was one grade 2 renal event (tubular acidosis)
Serious Adverse Events Through 96 Weeks
| ATV/r | LPV/r | | SAEs, N (%) | 16 (13) | 13 (11) | | SAES 1% or more, N (%) | | Infection | 7 (6) | 3 (2) | | Injury | 3 (3) | 6 (5) | | GI disorder | 2 (2) | 3 (2) | | Renal disorder | 0 | 3 (2) | | Neoplasm | 0 | 2 (2) | | Vascular disorder | 0 | 2 (2) |
Grade 3-4 Lab Abnormalities Through 96 Weeks
| ATV/r | LPV/r | | ALT elevation | 5% | 3% | | AST | 3% | 4% | | Total bilirubin elevation | 53% | <1% | | Neutropenia | 8% | 10% | | Thrombocytopenia | 5% | 5% |
No discontinuation due to elevated bilirubin
No grade 3-4 creatinine elevations
USE OF LIPID LOWERING AGENTS
PRIOR USE:
6% ATV/r
5% LPV/r
WEEK 48:
8% ATV/r
19% LPV/r
WEEK 96:
9% ATV/r
20% LPV/r
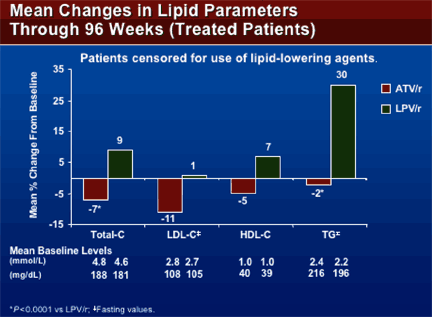
MEAN % CHANGES IN LIPIDS THROUGH 96 WEEKS
(treated patients), patients censored for using lipid-lowering agents
MEAN BASELINE LEVELS
| TOTAL CHOL: | | | | -7% ATV/r | 188 mg/dL | 4.8 mmol/L | | +9% LPV/r | 181 | 4.6 | | LDL-CHOL: | | | | -11% ATV/r | 108 mg/dL | 2.8 mmol/L | | +1% LPV/r | 105 | 2.7 | | HDL-CHOL: | | | | -5% ATV/r | 40 mg/dl | 1.0 mmol/L | | +7% LPV/r | 39 | 1.0 | | Triglycerides: | | | | -2% ATV/r | 216 mg/dL | 2.4 mmol/L | | +30% LPV/r | 196 | 2.2 |
|
|
| |
| |
 |
|
| |
| |
% of patients Using of Antidiarrheal Agents
PRIOR USE:
ATV/r: 0%
LPV/r: 5%
WEEK 48:
6% ATV/r
24% LPV/r
WEEK 96:
6% ATV/r
25% LPV/r
|
| |
|
 |
 |
|
|