 |
 |
 |
| |
INDEPENDENT VALIDATION AND COMPARISON WITH
FIBROSCAN, FIBROTEST AND LIVER BIOPSY OF CLINICAL
GLYCOMICS FOR THE NON INVASIVE ASSESSMENT OF
LIVER FIBROSIS IN CHRONIC HCV
|
| |
| |
Reported by Jules Levin
http://www.natap.org
from AASLD
Nov 11, 2005, San Francisco
Laurent Castera, Department of Hepatology, Hopital Haut Leveque, CHU Bordeaux, France, Pessac; Annelies Van Hecke, Department of Molecular
Biomedical Research, Ghent University and VIB, Zwijnaarde, Belgium; Pascale Trimoulet, Department of Virology, Hopital Pellegrin, CHU Bordeaux, Bordeaux, France; Nico Callewaert, Department of Molecular Biomedical Research, Ghent University and VIB,, Zwijnaarde, Belgium; Patrice Couzigou, Victor de Ledinghen, Department of Hepatology, Hopital Haut Leveque, CHU Bordeaux,
Pessac, France; Roland Contreras, Wouter Laroy, Department of Molecular Biomedical Research, Ghent University and VIB,, Zwijnaarde, Belgium
Background and Aims: Recently, novel and promising non invasive methods have been proposed for the assessment of liver fibrosis in chronic hepatitis C (CHC): “clinical glycomics”, based on DNA sequencer/fragments analysers to profile serum protein N-glycans; liver stiffness measurement, using FibroScan (FS, Echosens, France) and Fibrotest (FT, Biopredictive, France).
The aims of this study were: 1) to validate independently the diagnostic
performances of clinical glycomics in measuring liver fibrosis as compared to liver biopsy in patients with chronic hepatitis C; 2) to compare and combine its diagnostic performances to those of other non invasive markers (FS and FT), performed the same day as liver biopsy.
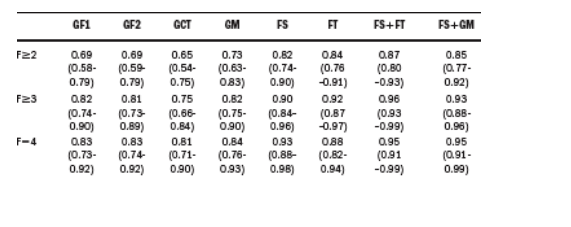
Methods: 111 CHC patients (57 males, mean age 53 12 years) were studied. Fibrosis was scored according to METAVIR by two independent pathologists blinded to FS and FT results. N-glycan occurrence on serum glycoproteins was assessed by DSA-FACE as previously described (Callewaert et al. Nat Med 2004), resulting in 3 diagnostic markers: GlycoFibro1 (GF1), GlycoFibro2 (GF2)
and GlycoCirrhoTest (GCT) and the combination of these 3 markers refered as combined model GlycoMarker (GM). Diagnostic performances were assessed using areas under the receiving operating characteristic curve (AUROC).
Results:
The fibrosis score according to Metavir split as follow: F0-F1 n=28, F2 n=30, F3 n=25, F4 n=28. The AUROCs (95% CI) are shown in the table:
Conclusions: Diagnostic performance of clinical glycomics appeared better for cirrhosis than for significant fibrosis (F>/=2), being of the same order as that of FS and FT. The combination of FS with FT and FS with glycomics allowed to yield the best perfomances. The use of the combination of FS with FT or with glycomics could be useful in clinical practice for non invasive assessment of liver
fibrosis in patients wtih CHC.
|
|
| |
| |
|
 |
 |
|
|