 |
 |
 |
| |
VX-950 Hep C Protease Inhibitor
|
| |
| |
"Final results of a phase 1b Multiple Dose Study of VX-950, a Hepatitis C Virus Protease Inhibitor"
Reported by Jules Levin
http://www.natap.org
from AASLD
Nov 14, 2005, San Francisco
Viral load reduction at day 14 was mean -4.4 log with the 750 mg every 8 hours dose.
HW Reesnik reported these data today at AASLD oral session. VX950 Ki=7nM.
Dose escalation study. Primary objective: assessment of safety. Secondary objective: assessment of PK and viral kinetics.
PART A: 24 healthy subjects. First study reported previously.
PART B: 36 subjects with genotype 1. These data were reported today.
DOSING: 450 mg or 750 mg every 8 hours, 1250 mg every 12 hours, or placebo for 14 days. In each cohort 2 placebo & 10 patients receiving VX-950.
Baseline Characteristics
Median weight 70-77 kg. Mostly treatment-experienced patients. Median HCV RNA: 6.38-6.48 log. Males & females.
Reesnik reported VX-950 was well tolerated. No serious adverse events. No treatment discontinuations. Most common AEs considered possibly related to study drug (all mild in severity): placebo n=6; VX-950 n=28
Headache: 33% placebo; 39% VX-950
Diarrhea: 17% placebo; 21% VX-950
Abdominal pain: 0% placebo; 14% VX-950
Nausea: 0% placebo; 14% VX-950
Dry mouth: 33% placebo; 11% VX-950
Flatulence: 33% placebo; 11% VX-950
Frequent urination: 33% placebo; 11% VX-950
Viral Load Reductions
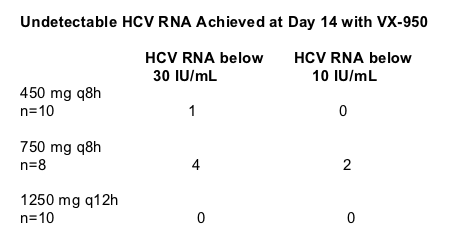
All patients on VX-950 had at least a 2 log drop in HCV RNA. The dose group of 750 mg every 8 hours had the best results:
(n=8): 3 patients had -3 to -4 log HCV RNA reductions; 3 patients had -4 to -5 log reductions. 2 patients had 5 log or more in viral load reductions.
Placebo (n=6): 6 patients had 0 to -1 log reduction.
450 mg every 8 hours (n=10): 1 patient had -2 to -3 log drop. 7 patients had -3 to -4 log drop. 2 patients had 5 log or more drop.
1250 mg every 12 hours (n=10); 1 patient had -2 to -3 log drop. 9 patients had -3 to -4 log drop.
By week 3 all three dose groups had 3 log viral load reduction, study authors called it " rapid & dramatic decreases". At week 3 the 750 mg dose group continued decrease in viral load with a steady decline to about a -4.4 log decrease at day 14. Phase 2 viral load decline was slow but steady & at day 14 the slope was still declining. But for the other two dose groups viral load started to increase at week 3.
The mean trough concentrations of VX-950 was 1054 ng/mL at day 14 for patients taking the 750 mg dose, 781 ng/mL for patients taking 1250 mg dose, and 676 ng/mL for patients taking the 450 mg dose.
Pharmacokinetic/Pharmacodynamic Analysis
VX-950 induced a biphasic decline in viral load.
VX-950 AUC during early dosing, and trough concentrations at steady state, were the key determinants of the first & second slope of viral load decline, respectively.
Viral kinetic modeling with extrapolation of antiviral responses indicates that viral eradication with VX-950 monotherapy could potentially occur in 12 weeks of dosing, the presenter said.
Viral Sequencing Analysis
The error prone RNA polymerase of HCV creates a variety of quasispecies, some of which may have decreased sensitivity to direct antivirals and can be selected during dosing.
A state-of-the-art viral sequencing approach suggests that suboptimal responses observed in some patients can be explained by selection of viral variants that contain 1 or 2 sequence changes at 4 specific locations in the protease region.
While exhibiting varying levels of reduced sensitivity to VX-950, these variants also demonstrated decreased replicative capacity.
|
|
| |
| |
|
 |
 |
|
|