 |
 |
 |
| |
SCH 503034 HCV Protease Inhibitor Monotherapy in HCV Genotype 1 IFN Nonresponders
|
| |
| |
Reported by Jules Levin
http://www.natap.org
from AASLD
Nov 14, 2005, San Francisco
Stefan Zeuzem reported the first study data in patients on this new HCV protease inhibitor. This was a double-blind, placebo-controlled, 14-day dosing study. The study population was IFN plus or minus ribavirin nonresponders, defined as having had <2 log decline after 12 weeks or more of therapy with interferon. Baseline HCV RNA was 30,000 IU/mL or more. Patients had compensated liver disease. 10-12 patients received study drug at each dose level & 4 received placebo. Doses of drug were 100, 200, and 400 mg twice daily, and 400 mg three times daily.
Zeuzem Summary:
Oral capsules were rapidly absorbed with dose-dependent increases in Cmax & AUC. Average HCV RNA (viral load reduction) with highest dose used in study, 400 mg 3 times daily, was about 1.6 log at day 14. Exposure & antiviral activity was dose related. Tolerability was similar to placebo. No phenotypic resistance was reported. A larger study is ongoing exploring utility of the drug.
Baseline Characteristics
Mostly males, but some women in study. Mean age was 40-53 yrs. HCV RNA (viral load) was 6.25 log IU/mL. Mean ALT was 82-112.
Pharmacokinetics
Of note, 100 mg capsules of SCH 503034 were used in this study. The study authors reported the drug was rapidly absorbed (Tmax about 1-2 hours). There was a dose-related increase in Cmax & AUC. There was a biphasic clearance (T halflife 7-15 hours. IC90: 400 nM in replicon assay.
Mean Decrease in HCV RNA
At day two the maximal mean HCV RNA decrease was reached with the highest dose & it was about 1.8 log, but mean HCV RNA appeared to be about 1.6 log at day 14. The mean HCV RNA decline for the 200 & 400 mg dose groups was about 0.80 log at day 14.
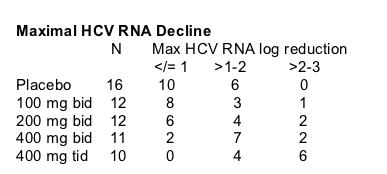
Declines in HCV RNA appeared to be associated with serum drug concentrations.
Mean ALT reductions from baseline was -55 with the 400 mg tid, highest dose, but less with the other doses.
Sequence Analysis of HCV Protease
42/43 patients had no emergence of resistant variants. A single variant, V170A, which causes resistance in vitro, was identified in 1 patient. The variant became nondetectable during followup.
SCH503034 Safety
The drug was well tolerated.
No dose related increase in adverse events were reported.
Most frequent adverse event: headache (12% vs 29% in placebo).
AEs were reported mild or moderate & not different from placebo.
Clinical lab values were reported not different from placebo.
No increases reported in bilirubin, creatinine, prothrombin time.
No clinically significant ECG change was reported.
|
|
| |
| |
|
 |
 |
|
|