 |
 |
 |
| |
Entecavir 2 Years Study Results in HBeAg+ HBV+ Patients (study ETV-22)
|
| |
| |
Reported by Jules Levin
http://www.natap.org
from AASLD
Nov 15, 2005, San Francisco
"Entecavir Results in substantial Virologic and Biochemical Improvement and HBeAg Seroconversion Through 96 Weeks of treatment in HBeAg+ Chronic Hepatitis B Patients (Study ETV-022)"
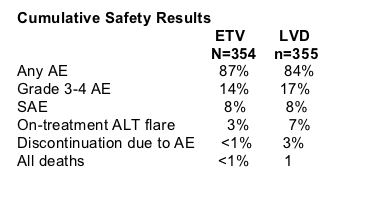
A cumulative total of 80% of study participants receiving entecavir during first year & second year in Study ETV-022 achieved <300 copies/ml compared to 39% for patients in study receiving lamivudine. Cumulative confirmed results for all treated patients show 31% HBeAg seroconversion for patients receiving entecavir & 26% for patients receiving lamivudine. 79% of patients taking entecavir normalized ALT through the 2nd year compared to 68% for patients taking lamivudine. After 96 weeks treatment in the study 8% (n=27) of patients receiving entecavir were considered nonresponders compared to 41% (n=147) for patients taking lamivudine (Npnresponder defined as >/= 0.7 MEqmL by bDNA. Safety profile for the two drugs is comparable. The authors reported that lamivudine treatment beyond 48 weeks showed no benefit by HBV DNA or ALT normalization: 30% with HBV DNA >/= 100,000 copies/ml.
Week 52 Patient Management Decision
Definitions:
Response -R-: HBV DNA <0.7 MEqmL by bDNA & HBeAg loss
Virologic response -VR- : HBV DNA <0.7 MEqmL by bDNA and HBeAG+
Non-response -NR- : HBV DNA >/=0.7 MEqmL by bDNA
ETV (n=354)
R (n=74) these patients were discontinued from study after 48 weeks
VR (n=247) these patients continued in the study and are the subject of the analysis
NR (n=19) these patients discontinued study
Missing (n=14)
Lamivudine (LVD) n=355
R (n=67) discontinued at wk 48
VR (n=165) these pts continued in study through week 96
NR (n=94) discontinued
Missing (n=29)
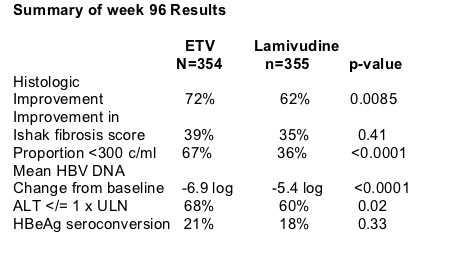
RESULTS
Response Outcomes Through Week 96
EVT
VR=247, 243 continue to year 2, VR=198, R=37, NR=8
LVD
VR=165, 164 continue to year 2, VR=85, R=26, NR=53
Year 2 Treatment Cohort: Proportion with HBV DNA <300 copies/ml at week 48 & through Week 96
ETV
Week 48: 64% <300 c/ml
End of Dosing (defined as the last observation on-treatment): 81%
LVD
Week 48: 40% <300 c/ml
EOD: 39% <300 c/ml
Year 2 Treatment Cohort:
ALT
ETV
Week 48: 66%
EOD: 79%
LVD
Week 48: 71%
EOD: 68%
All Treated Patients: Cumulative Confirmed results
ETV: n=354
LVD: n=355
Cumulative proportion of treated subjects who ever achieved a confirmed endpoint.
Confirmed - 2 sequential measurements meet success criteria (or last observation).
All treated Patients: cumulative response outcomes up to 96 weeks
ETV=354
80% <300 copies/ml HBV DNA
111 (31%) responder
202 (57% Virologic Responder
27 (85) Non-responder
LVD=355
39% <300 copies/ml HBV DNA
93 (26%) responder
86 (24%) Virologic responder
147 (41%) nonresponder
All Treated Patients: Cumulative Confirmed HBeAg Seroconversion & HBsAg loss
HBeAg Seroconversion
31% ETV (n=354)
26% LVD (n=355)
HBsAg loss
5% ETV (18/354)
3% LVD (10/355)
Responders: 24 weeks off-treatment
Response is defined as HBV DNA <0.7 MEq/mL by bDNA & loss of HBeAg.
ETV n=111
LVD n=93
Response:
76% ETV
72% LVD
ALT
63% LVD
HBeAg seroconversion:
71% ETV
69% LVD
HBV DNA <300 copies/ml:
31% ETV
29% LVD
|
|
| |
| |
|
 |
 |
|
|