 |
 |
 |
| |
NM283 +Pegasys for Nonresponders: phase II 12 weeks
|
| |
| |
Reported by Jules Levin
http://www.natap.org
AASLD Nov 2005, San Francisco
"Randomized Trial of Valopicitabine (NM283) Alone and in Combination with Peg-Interferon vs. Retreatment with Peg-Interferon plus Ribavirin (PegIFN/RBV) in Hepatitis C Patients with Previous Non-Response to PegIFN/RBV: First Interim Results"
Phase IIb Trial ongoing:12 week interim analysis
C. O'Brien, E. Godofsky, M. Rodriguez-Torres, N. Afdhal, S. Pappas, P. Pockros, E. Lawitz, N. Bzowej, V. Rustgi, M. Sulkowski, K. Sherman, I. Jacobson, G. Chao, S. Knox, K. Pietropaolo, and N. Brown
AASLD 2005 Annual Meeting
San Francisco, CA
Nov. 14, 2005
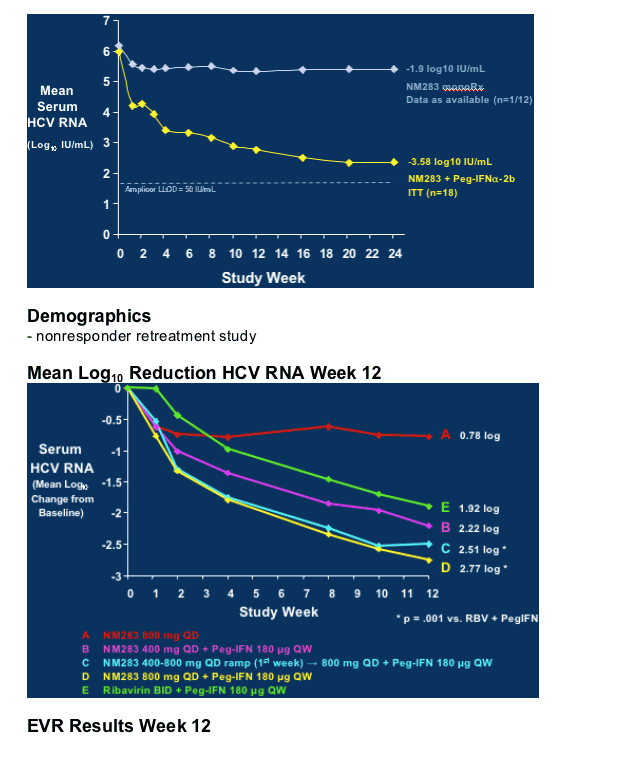
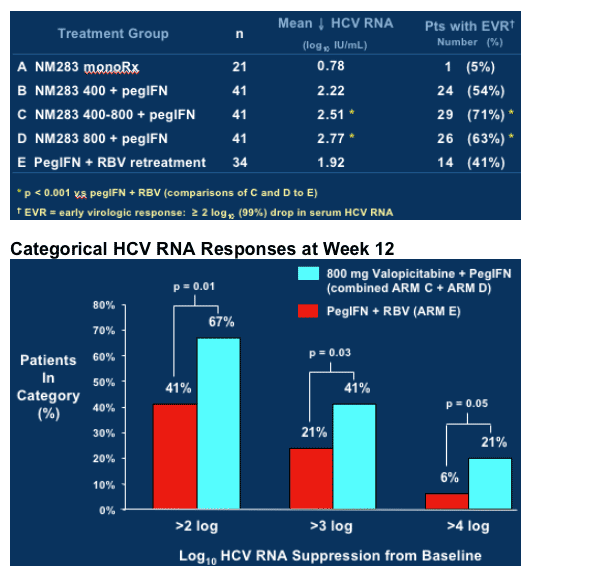
As you can see in first graph, in treatment-naive study, after 24 weeks NM283 monotherapy resulted in -1.9 logIU/mL HCV RNA reduction, and in combination with peginterferon HCV RNA reduction was -3.58 log IU/mL. In phase IIb study in peginterferon/RBV nonresponders HCV RNA reduction was -0.78 for highest dose of NM283 800mg/day (4 200mg tablets) after 12 weeks, and for combination therapy with peginterferon plus NM283 800mg/day the HCV RNA reduction after 12 weeks was -2.77 log. Retreatment with Pegasys plus ribavirin (1000-1200mg/day) reduced HCV RNA by -1.92 log in the study after 12 weeks.
NM283 Phase IIa Trial, Treatment-Naive Patients
Serum HCV RNA Levels, Baseline to Week 24
Safety
4% (n=7) discontinuations for various adverse events by Week 12
--No predominant treatment-limiting AE or lab abnormality
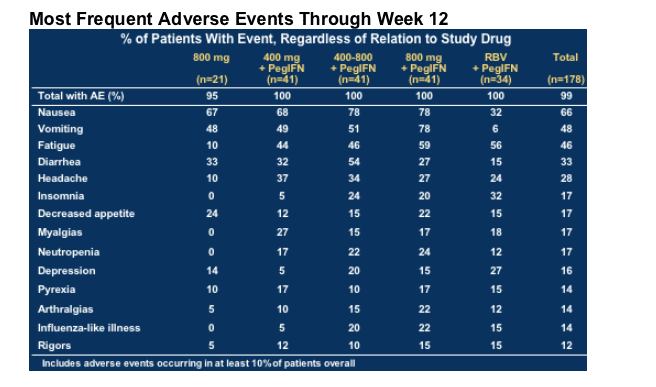
GI symptoms affect proportionally more patients on NM283 + pegIFN
--Usually self-limiting and usually tolerable
Serious adverse events (SAE) in 6/178 patients (3%) through Week 12
--Two attributed to study medication (anemia, dehydration)
Four for GI symptoms; some not attributed to study treatment (diverticulitis)
--All SAEs have resolved
No hematologic toxicities (grade 1-4) for NM283 monotherapy; neutropenia and thrombocytopenia noted in all arms with pegIFN
Sporadic elevations of amylase, lipase, AST, ALT
--Variable, usually mild (grade 1 & 2), rarely treatment-limiting
Most Frequent Adverse Events Through Week 12
Valopicitabine (NM283) Phase IIb
Non-Responder Trial: Valopicitabine
NS5b polymerase inhibitor
- Ribonucleoside
- NM107-triphosphate competitively inhibits viral polymerase and is incorporated into viral RNA, causing chain termination
Oral agent
- Valyl ester pro-drug provides high oral bioavailability
- Plasma half life (4-6 hrs) & intracellular half life (15 hrs) support once daily dosing
Valopicitabine (NM283) Phase IIb
Non-Responder Trial: Aims
To compare safety, tolerability and antiviral activity of:
--NM283 monotherapy
--NM283 + PegIFN alfa-2a
--Retreatment with PegIFN alfa-2a + ribavirin
In patients with genotype 1 chronic hepatitis C who were nonresponders to PegIFN + ribavirin
Valopicitabine (NM283) Phase IIb
Non-Responder Trial: Inclusion Criteria
18-65 years of age, male or female
HCV genotype 1
Nonresponders defined as adequate treatment course
- At least 12 weeks of pegIFN alfa + RBV
- At least 75% of the prescribed doses of pegIFN and ribavirin
- Failed to clear HCV RNA to non-detectable levels
- Patients must have previously failed for efficacy, not safety
Baseline
--HCV RNA > 105 IU/mL (100,000 IU/mL)
--ALT L 5 x ULN
--Compensated liver disease
Study Design
Multicenter (22), randomized, active control design
HCV RNA response criteria at Weeks 4, 12, 24:
--Patients required to have HCV RNA drop ≥ 0.5 log at Week 4,
≥ 1.0 log at Week 12, and > 2.0 log at Week 24 to continue treatment
--Early termination if viral response criteria not met
Primary efficacy endpoint: ITT analysis will be the SVR 6 months after the last dose of study medicine:
--HCV RNA: COBAS TaqMan PCR assay (10 IU/mL; Roche)
--NM283 dosing groups pooled and compared to PegIFN + ribavirin
Non-Responder Trial: Treatment Design
171 patients to be randomized in a 1:2:2:2:2 ratio
--NM283 monotherapy, 800 mg/day (19 pts)
--NM283 400 mg/day + pegIFNa-2a (38 pts)
--NM283 400AE800 mg/day + pegIFNa-2a (38 pts)
--NM283 800 mg/day + pegIFNa-2a (38 pts)
--PegIFNa-2a + RBV (38 pts)
Medication dosing
--NM283 200 mg tablet
--PegIFN-a2a (180 mg SQ weekly) started on Day 8 for all pegIFN-a2a treatment regimens
--Ribavirin < 75 kg :1000 mg/d ; > 75 kg : 1200 mg/d
Current Status
190 patients were randomized (fully enrolled)
--12 patients withdrew prior to receiving study medications
--9 withdrew consent after randomization to Peg-IFN/RBV
--3 withdrew consent for other reasons
178 patients received study medications
--21 patients discontinued by week 12
5 withdrew consent for other reasons
9 virologic non-response at Week 4
7 discontinued for adverse events
157 patients past week 12
--Trial ongoing:12 week interim analysis
|
|
| |
| |
|
 |
 |
|
|