 |
 |
 |
| |
24 Week Pegasys+RBV Treatment SVR in Genotype 1 Predicted by Wk 4 Response
|
| |
| |
"Rapid virological response at week 4 of peginterferon alfa-2a (40KD) (PEGASYS) plus ribavirin (COPEGUS) treatment predicts sustained virological response after 24 weeks in genotype 1 patients"
Reported by Jules Levin
Presented at the 56th Annual Meeting of the American Association for the Study of Liver Diseases: 2005 November 11- 15: San Francisco, CA, USA.
This research was funded by Roche, Basel, Switzerland.
D. Jensen,1 T.R. Morgan,2 P. Marcellin,3 P.J. Pockros,4 K.R. Reddy,5 S.J. Hadziyannis,6 P. Ferenci,7 B. Willems8
1University of Chicago Hospitals, Chicago, IL, USA; 2VA Medical Center, Long Beach, CA, USA; 3Hôpital Beaujon, Clichy, France; 4The Scripps Clinic, La Jolla CA, USA; 5University of Pennsylvania, Philadelphia, PA, USA; 6Henry Dunant Hospital, Athens, Greece;
7Department of Internal Medicine IV, Medical University of Vienna, Austria; 8Centre Hospitalier de l'Université de Montréal, Montréal QC, Canada
AUTHOR'S CONCLUSIONS
This retrospective analysis of a large, randomized, phase III trial including
729 genotype 1 patients[4] shows that:
--Approximately 20% of patients with HCV genotype 1 infection achieved
undetectable HCV RNA levels by qualitative PCR (i.e. an RVR) at week 4
of treatment with peginterferon alfa-2a (40KD) (PEGASYS®) plus ribavirin
(COPEGUS).
--Overall, 89% of patients with an RVR treated for only 24 weeks achieved
an SVR. Moreover, patients with an RVR treated for 48 weeks did not obtain
a better SVR rate.
--The highest SVR rate in patients without an RVR (44%) was obtained following
treatment with peginterferon alfa-2a (40KD) plus ribavirin at the recommended
dose (1000/1200 mg/day) for the recommended duration (48 weeks).
--A lower baseline HCV RNA level was the only significant and independent
factor associated with an RVR. (>600,000 IU/ml did not perform as well, see table below)
--An RVR (<50 IU/ml) at week 4 of treatment is the single best predictive factor for an SVR.
The use of RVR status to guide treatment duration in genotype 1 patients is
appealing and should ideally be confirmed by prospective studies.
INTRODUCTION
The recommended treatment for patients with hepatitis C virus (HCV) genotype 1
infection is the combination of a pegylated interferon plus ribavirin 1000 or
1200 mg/day for 48 weeks.[1,2] The ability to identify genotype 1 patients that might respond to a reduced length of treatment would limit the burden of therapy in this common but more difficult-to-treat patient population. Recent data from an uncontrolled study suggest that HCV genotype 1 patients with a low baseline viral load who have undetectable HCV RNA after 4 weeks of treatment with the combination of a pegylated interferon plus ribavirin may require just 24 weeks of treatment to achieve a sustained virological response (SVR).[3]
In a randomized, phase III trial by Hadziyannis et al.[4] that examined treatment duration and ribavirin dose, 78 out of 219 (36%) patients with HCV genotype 1 infection achieved an SVR after 24 weeks of treatment with peginterferon alfa-2a (40KD) (PEGASYS) plus ribavirin (COPEGUS). We analyzed data from patients with genotype 1 infection in this trial.
OBJECTIVE
The aim of this study was to retrospectively examine whether a rapid virological
response (RVR) at week 4 of treatment was predictive of an SVR in HCV genotype 1 patients receiving peginterferon alfa-2a (40KD) plus ribavirin for 24 weeks in a randomized, multinational, phase III trial.[4]
The study also attempted to identify factors predictive of an RVR in patients randomized to 24 weeks of treatment.
METHODS
Patients
Patients included in the study were treatment-naive adults aged ≥18 years with
quantifiable serum HCV RNA levels, evidence of liver disease consistent with chronic hepatitis C and elevated alanine aminotransferase (ALT) activity in serum. Exclusion criteria included the presence of neutropenia, thrombocytopenia, anemia or a serum creatinine level ≥1.5 times the upper limit of normal. Patients co-infected with hepatitis A or B virus or HIV, and those with severe psychiatric illnesses or clinically significant coexisting medical conditions were also excluded.
Study design
Patients were randomized to one of four groups that differed by treatment duration and ribavirin dose. All patients received subcutaneous peginterferon alfa-2a (40KD) 180 _g/week in combination with:
- low-dose (LD [800 mg/day]) ribavirin for 24 (24-LD) or 48 weeks (48-LD)
- standard-dose (SD [1000 mg/day for bodyweight <75 kg, 1200 mg/day for
bodyweight ≥75 kg]) ribavirin for 24 (24-SD) or 48 weeks (48-SD).
The primary efficacy end-point of the trial was SVR, defined as undetectable (<50 IU/mL) serum HCV RNA by qualitative polymerase chain reaction (PCR) assay (COBAS AMPLICOR HCV Test, v2.0) at the end of a 24-week untreated follow-up period. RVR was defined as undetectable HCV RNA (<50 IU/mL) by qualitative PCR at week 4.
Statistical analysis
Stepwise multiple regression analysis was used to explore prognostic factors for an RVR. Baseline disease and demographic factors considered for entry included age, gender, bodyweight, body mass index, body surface area, log10 baseline HCV RNA level, histological diagnosis, baseline ALT activity, qualifying ALT quotient, genotype 1 subtype and ribavirin dose at baseline.
RESULTS
In total, 740 patients infected with HCV genotype 1 were randomized to treatment and received at least one dose of study medication. Week 4 virological test results were available in 729 of these patients, 146 of whom achieved an RVR. A total of 216 patients received treatment for 24 weeks. Of these, 51 (24%) achieved an RVR. Baseline disease and demographic characteristics according to RVR status in patients randomized to 24 weeks of treatment are presented in Table 1.
Table 1. Baseline disease and demographic characteristics according to PCR status at week 4 in genotype 1 patients randomized to 24 weeks of treatment
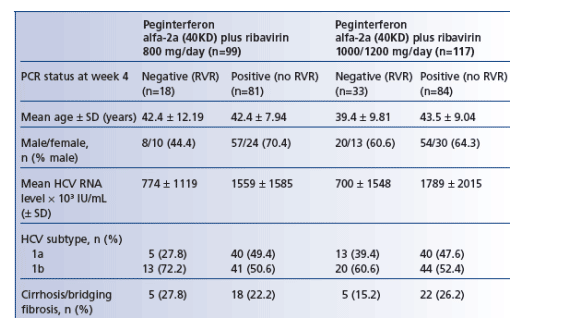
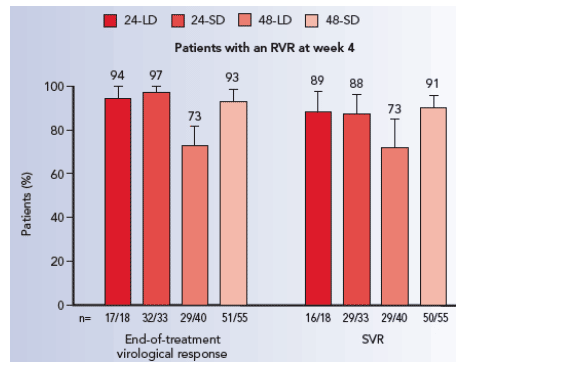
- In all four treatment groups, patients with an RVR achieved higher end-of-treatment virological response rates and higher SVR rates than patients without an RVR. SVR rates in patients with an RVR were generally similar across all four treatment groups (Figure 1).
- In patients randomized to treatment for 24 weeks, overall SVR rates were markedly higher in patients who did (89%) vs did not (19%) achieve an RVR.
- Patients with an RVR had lower relapse rates between end of treatment and followup than those without an RVR, irrespective of treatment duration or ribavirin dose.
- In contrast, there were obvious trends towards decreased relapse rates in patients without an RVR when treatment was given for 48 weeks and also when standard dose ribavirin was given.
- 75% and 68% of patients without an RVR relapsed following peginterferon alfa-2a (40KD) plus ribavirin 800 and 1000/1200 mg/day for 24 weeks
- 42% and 32% of patients without an RVR relapsed following peginterferon alfa-2a (40KD) plus ribavirin 800 and 1000/1200 mg/day for 48 weeks.
Multiple regression analyses
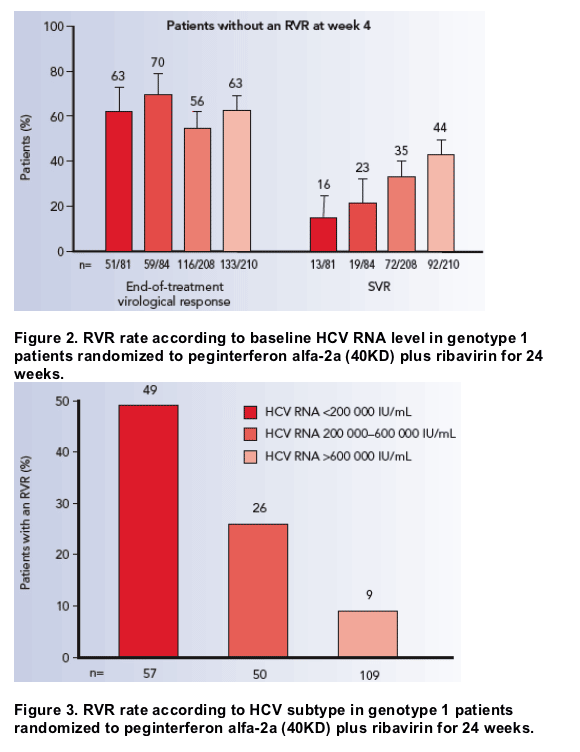
Multiple regression analysis showed that a lower baseline HCV RNA level was
predictive of an RVR in patients treated for 24 weeks. Patients with a baseline
HCV RNA level of <200 000 IU/mL (OR 9.7; 95% CI 4.2- 22.5; p<0.0001) or
200 000- 600 000 IU/mL (OR 3.6; 95% CI 1.5- 9.1; p=0.0057) were significantly
more likely to achieve an RVR than patients with a baseline HCV RNA level
>600 000 IU/mL (Figure 2).
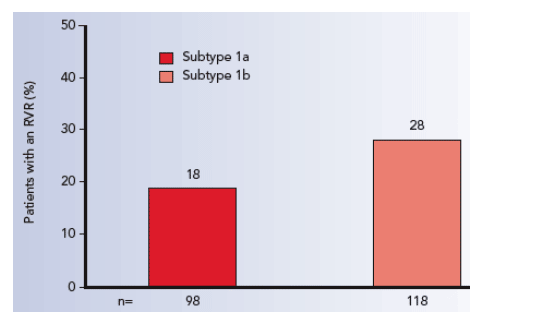
An RVR was more likely in subtype 1b vs 1a patients (OR 1.8; 95% CI 0.9- 3.7;
p=0.095) (Figure 3). In subtype 1a patients a baseline HCV RNA level of
≤200 000 vs >200 000 IU/mL was significantly associated with an RVR (OR 5.9;
95% CI 2.0- 17.6; p=0.0015), while in genotype 1b patients a baseline HCV RNA
of ≤600 000 vs >600 000 IU/mL was significantly associated with an RVR (OR 10.3; 95% 3.6- 29.4; p<0.0001).
Figure 1. End-of-treatment virological response rate and SVR rate according to RVR status in genotype 1 patients receiving peginterferon alfa-2a (40KD) plus ribavirin.
REFERENCES
1. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements 2002; 19: 1- 46
2. Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology 2004; 39: 1147- 71
3. Zeuzem S, Esteban-Mur R, Buti-Ferrer M, et al. Efficacy of 24 weeks of treatment with peginterferon _-2b 1.5 _g/kg/week plus ribavirin 800- 1400 mg/day in patients infected with chronic hepatitis C genotype 1 of low viral load (LVLG1). Poster presented at Digestive Disease Week. Chicago, IL USA, May 2005
4. Hadziyannis S, Sette H, Morgan T, et al. Peginterferon-alpha- 2a and ribavirin
combination therapy in chronic hepatitis C: a randomized study of treatment
duration and ribavirin dose. Ann Intern Med 2004; 140: 346- 55
|
|
| |
| |
|
 |
 |
|
|